Infectious Virus Persists in CD4+ T Cells and Macrophages in Antiretroviral Therapy-Suppressed Simian Immunodeficiency Virus-Infected Macaques
- PMID: 31118264
- PMCID: PMC6639293
- DOI: 10.1128/JVI.00065-19
Infectious Virus Persists in CD4+ T Cells and Macrophages in Antiretroviral Therapy-Suppressed Simian Immunodeficiency Virus-Infected Macaques
Abstract
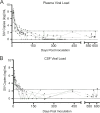
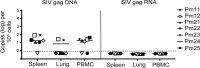
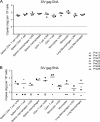
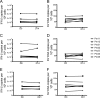
Understanding the cellular and anatomical sites of latent virus that contribute to human immunodeficiency virus (HIV) rebound is essential for eradication. In HIV-positive patients, CD4+ T lymphocytes comprise a well-defined functional latent reservoir, defined as cells containing transcriptionally silent genomes able to produce infectious virus once reactivated. However, the persistence of infectious latent virus in CD4+ T cells in compartments other than blood and lymph nodes is unclear. Macrophages (Mϕ) are infected by HIV/simian immunodeficiency virus (SIV) and are likely to carry latent viral genomes during antiretroviral therapy (ART), contributing to the reservoir. Currently, the gold standard assay used to measure reservoirs containing replication-competent virus is the quantitative viral outgrowth assay (QVOA). Using an SIV-macaque model, the CD4+ T cell and Mϕ functional latent reservoirs were measured in various tissues using cell-specific QVOAs. Our results showed that blood, spleen, and lung in the majority of suppressed animals contain latently infected Mϕs. Surprisingly, the numbers of CD4+ T cells, monocytes, and Mϕs carrying infectious genomes in blood and spleen were at comparable frequencies (∼1 infected cell per million). We also demonstrate that ex vivo viruses produced in the Mϕ QVOA are capable of infecting activated CD4+ T cells. These results strongly suggest that latently infected tissue Mϕs can reestablish productive infection upon treatment interruption. This study provides the first comparison of CD4+ T cell and Mϕ functional reservoirs in a macaque model. It is the first confirmation of the persistence of latent genomes in monocytes in blood and Mϕs in the spleen and lung of SIV-infected ART-suppressed macaques. Our results demonstrate that transcriptionally silent genomes in Mϕs can contribute to viral rebound after ART interruption and should be considered in future HIV cure strategies.IMPORTANCE This study suggests that CD4+ T cells found throughout tissues in the body can contain replication-competent SIV and contribute to rebound of the virus after treatment interruption. In addition, this study demonstrates that macrophages in tissues are another cellular reservoir for SIV and may contribute to viral rebound after treatment interruption. This new insight into the size and location of the SIV reservoir could have great implications for HIV-infected individuals and should be taken into consideration for the development of future HIV cure strategies.
Keywords: CD4+ T cells; latency; macrophages; quantitative viral outgrowth assay; reservoir; simian immunodeficiency virus.
Copyright © 2019 Abreu et al.
Figures




References
-
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. doi:10.1126/science.278.5341.1295. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

