Recapitulation of HDV infection in a fully permissive hepatoma cell line allows efficient drug evaluation
- PMID: 31118422
- PMCID: PMC6531471
- DOI: 10.1038/s41467-019-10211-2
Recapitulation of HDV infection in a fully permissive hepatoma cell line allows efficient drug evaluation
Abstract
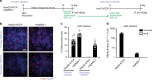
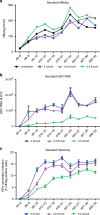
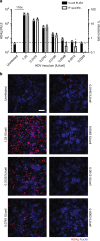
Hepatitis delta virus (HDV) depends on the helper function of hepatitis B virus (HBV), which provides the envelope proteins for progeny virus secretion. Current infection-competent cell culture models do not support assembly and secretion of HDV. By stably transducing HepG2 cells with genes encoding the NTCP-receptor and the HBV envelope proteins we produce a cell line (HepNB2.7) that allows continuous secretion of infectious progeny HDV following primary infection. Evaluation of antiviral drugs shows that the entry inhibitor Myrcludex B (IC50: 1.4 nM) and interferon-α (IC50: 28 IU/ml, but max. 60-80% inhibition) interfere with primary infection. Lonafarnib inhibits virus secretion (IC50: 36 nM) but leads to a substantial intracellular accumulation of large hepatitis delta antigen and replicative intermediates, accompanied by the induction of innate immune responses. This work provides a cell line that supports the complete HDV replication cycle and presents a convenient tool for antiviral drug evaluation.
Conflict of interest statement
S.U. is a co-applicant and co-inventor on patents protecting Myrcludex B as an HBV/HDV entry inhibitor. The remaining authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

