Form vision from melanopsin in humans
- PMID: 31118424
- PMCID: PMC6531428
- DOI: 10.1038/s41467-019-10113-3
Form vision from melanopsin in humans
Abstract
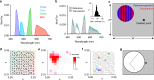
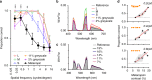
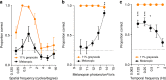
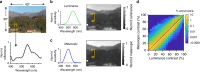
Detection and discrimination of spatial patterns is thought to originate with photoreception by rods and cones. Here, we investigated whether the inner-retinal photoreceptor melanopsin could represent a third origin for form vision. We developed a 4-primary visual display capable of presenting patterns differing in contrast for melanopsin vs cones, and generated spectrally distinct stimuli that were indistinguishable for cones (metamers) but presented contrast for melanopsin. Healthy observers could detect sinusoidal gratings formed by these metamers when presented in the peripheral retina at low spatial (≤0.8 cpd) and temporal (≤0.45 Hz) frequencies, and Michelson contrasts ≥14% for melanopsin. Metameric gratings became invisible at lower light levels (<1013 melanopsin photons cm-2 sr-1 s-1) when rods are more active. The addition of metameric increases in melanopsin contrast altered appearance of greyscale representations of coarse gratings and a range of everyday images. These data identify melanopsin as a new potential origin for aspects of spatial vision in humans.
Conflict of interest statement
A.E.A. and R.J.L. are listed as inventors on patent PCT/GB2017/050338: ‘Improvements in image formation’. F.P.M. declares no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

