Development of a novel multiphysical approach for the characterization of mechanical properties of musculotendinous tissues
- PMID: 31118478
- PMCID: PMC6531478
- DOI: 10.1038/s41598-019-44053-1
Development of a novel multiphysical approach for the characterization of mechanical properties of musculotendinous tissues
Abstract
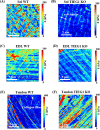
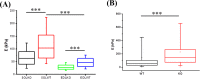
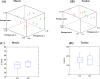
At present, there is a lack of well-validated protocols that allow for the analysis of the mechanical properties of muscle and tendon tissues. Further, there are no reports regarding characterization of mouse skeletal muscle and tendon mechanical properties in vivo using elastography thereby limiting the ability to monitor changes in these tissues during disease progression or response to therapy. Therefore, we sought to develop novel protocols for the characterization of mechanical properties in musculotendinous tissues using atomic force microscopy (AFM) and ultrasound elastography. Given that TIEG1 knockout (KO) mice exhibit well characterized defects in the mechanical properties of skeletal muscle and tendon tissue, we have chosen to use this model system in the present study. Using TIEG1 knockout and wild-type mice, we have devised an AFM protocol that does not rely on the use of glue or chemical agents for muscle and tendon fiber immobilization during acquisition of transversal cartographies of elasticity and topography. Additionally, since AFM cannot be employed on live animals, we have also developed an ultrasound elastography protocol using a new linear transducer, SLH20-6 (resolution: 38 µm, footprint: 2.38 cm), to characterize the musculotendinous system in vivo. This protocol allows for the identification of changes in muscle and tendon elasticities. Such innovative technological approaches have no equivalent to date, promise to accelerate our understanding of musculotendinous mechanical properties and have numerous research and clinical applications.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO.Sci Rep. 2018 Nov 20;8(1):17064. doi: 10.1038/s41598-018-34719-7. Sci Rep. 2018. PMID: 30459432 Free PMC article.
-
The association of muscle and tendon elasticity with passive joint stiffness: In vivo measurements using ultrasound shear wave elastography.Clin Biomech (Bristol). 2015 Dec;30(10):1230-5. doi: 10.1016/j.clinbiomech.2015.07.014. Epub 2015 Aug 14. Clin Biomech (Bristol). 2015. PMID: 26296832
-
Shear waves elastography for assessment of human Achilles tendon's biomechanical properties: an experimental study.J Mech Behav Biomed Mater. 2017 May;69:178-184. doi: 10.1016/j.jmbbm.2017.01.007. Epub 2017 Jan 6. J Mech Behav Biomed Mater. 2017. PMID: 28086149
-
Transmission of forces within mammalian skeletal muscles.J Biomech. 1999 Apr;32(4):371-80. doi: 10.1016/s0021-9290(98)00189-4. J Biomech. 1999. PMID: 10213027 Review.
-
Viscoelasticity and function of connectin/titin filaments in skinned muscle fibers.Adv Biophys. 1996;33:159-71. doi: 10.1016/s0065-227x(96)90031-3. Adv Biophys. 1996. PMID: 8922111 Review. No abstract available.
Cited by
-
A Shift in Tissue Stiffness During Hippocampal Maturation Correlates to the Pattern of Neurogenesis and Composition of the Extracellular Matrix.Front Aging Neurosci. 2021 Jul 30;13:709620. doi: 10.3389/fnagi.2021.709620. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34393762 Free PMC article.
-
Cytoplasmic anillin and Ect2 promote RhoA/myosin II-dependent confined migration and invasion.Nat Mater. 2025 Sep;24(9):1476-1488. doi: 10.1038/s41563-025-02269-9. Epub 2025 Jun 26. Nat Mater. 2025. PMID: 40571734 Free PMC article.
-
Expression of Myomaker and Myomerger in myofibers causes muscle pathology.Skelet Muscle. 2023 May 1;13(1):8. doi: 10.1186/s13395-023-00317-z. Skelet Muscle. 2023. PMID: 37127758 Free PMC article.
-
Interplay among cell migration, shaping, and traction force on a matrix with cell-scale stiffness heterogeneity.Biophys Physicobiol. 2022 Sep 13;19:e190036. doi: 10.2142/biophysico.bppb-v19.0036. eCollection 2022. Biophys Physicobiol. 2022. PMID: 36349327 Free PMC article.
-
Aerobic exercise and scaffolds with hierarchical porosity synergistically promote functional recovery post volumetric muscle loss.Biomaterials. 2023 May;296:122058. doi: 10.1016/j.biomaterials.2023.122058. Epub 2023 Feb 17. Biomaterials. 2023. PMID: 36841214 Free PMC article.
References
-
- Marturano JE, Arena JD, Schiller ZA, Georgakoudi I, Kuo CK. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6370–6375. doi: 10.1073/pnas.1300135110. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

