Improving medication adherence in adult kidney transplantation (IMAKT): A pilot randomised controlled trial
- PMID: 31118485
- PMCID: PMC6531445
- DOI: 10.1038/s41598-019-44002-y
Improving medication adherence in adult kidney transplantation (IMAKT): A pilot randomised controlled trial
Abstract
Resources to support long-term medication adherence in kidney transplantation are limited. This study aimed to determine the efficacy of an intervention designed for kidney transplant recipients to enhance medication adherence. A single-blind, multi-site, 12-month pilot randomised controlled trial was conducted at all five public hospitals providing adult kidney transplantation in Victoria, Australia. Participants were recruited at 4 to 6 weeks post-transplantation. Thirty-five participants were randomly assigned to a 3-month intervention, involving a face-to-face meeting (a medication review and a consumer-centred video) and health coaching every two weeks. Thirty-six were randomised to receive usual care. All participants were followed for nine months post-intervention. There were no differences in adherence between groups measured by Medication Event Monitoring System (MEMS), however, it was underutilised by 42% of participants. Based on the self-reported Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS©) score, the percentage of adherent participants decreased significantly between baseline and 3 to 12 months in the control group (p-values < 0.001) whilst the percentage of adherent participants in the intervention group remained constant over time. No group differences were detected in other outcomes. Due to the complex medication regimen, developing and testing a medication adherence intervention is difficult in kidney transplantation.
Conflict of interest statement
The authors declare no competing interests.
Figures
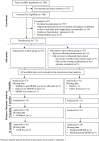

References
-
- Joost R, Dorje F, Schwitulla J, Eckardt KU, Hugo C. Intensified pharmaceutical care is improving immunosuppressive medication adherence in kidney transplant recipients during the first post-transplant year: A quasi-experimental study. Nephrol Dial Transplant. 2014;29:1597–1607. doi: 10.1093/ndt/gfu207. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

