Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls
- PMID: 31118516
- PMCID: PMC6699738
- DOI: 10.1038/s41586-019-1231-2
Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls
Abstract
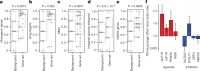
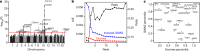
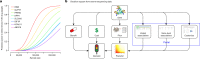
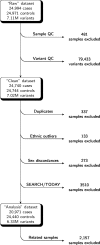
Protein-coding genetic variants that strongly affect disease risk can yield relevant clues to disease pathogenesis. Here we report exome-sequencing analyses of 20,791 individuals with type 2 diabetes (T2D) and 24,440 non-diabetic control participants from 5 ancestries. We identify gene-level associations of rare variants (with minor allele frequencies of less than 0.5%) in 4 genes at exome-wide significance, including a series of more than 30 SLC30A8 alleles that conveys protection against T2D, and in 12 gene sets, including those corresponding to T2D drug targets (P = 6.1 × 10-3) and candidate genes from knockout mice (P = 5.2 × 10-3). Within our study, the strongest T2D gene-level signals for rare variants explain at most 25% of the heritability of the strongest common single-variant signals, and the gene-level effect sizes of the rare variants that we observed in established T2D drug targets will require 75,000-185,000 sequenced cases to achieve exome-wide significance. We propose a method to interpret these modest rare-variant associations and to incorporate these associations into future target or gene prioritization efforts.
Conflict of interest statement
P.Z. is a consultant for Merck, Daichii-Sankyo, Boerhinger-Ingelheim and Janssen; B.M.P. serves on the DSMB of a clinical trial funded by Zoll LifeCor and on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson.
Figures








Comment in
-
Defining genetic risk of T2DM.Nat Rev Endocrinol. 2019 Aug;15(8):438. doi: 10.1038/s41574-019-0225-3. Nat Rev Endocrinol. 2019. PMID: 31160731 No abstract available.
-
T2D Risk Genes: Exome Sequencing Goes Straight to the Source.Cell Metab. 2019 Jul 2;30(1):10-11. doi: 10.1016/j.cmet.2019.06.010. Cell Metab. 2019. PMID: 31269421
References
MeSH terms
Grants and funding
- N01 HC095161/HL/NHLBI NIH HHS/United States
- K01 HL125751/HL/NHLBI NIH HHS/United States
- M01 RR000043/RR/NCRR NIH HHS/United States
- L30 DK106874/DK/NIDDK NIH HHS/United States
- R01 DK093757/DK/NIDDK NIH HHS/United States
- U01 HG007417/HG/NHGRI NIH HHS/United States
- R01 AG042188/AG/NIA NIH HHS/United States
- RC2 HL102419/HL/NHLBI NIH HHS/United States
- K24 DK110550/DK/NIDDK NIH HHS/United States
- U01 DK061254/DK/NIDDK NIH HHS/United States
- N01 HC085080/HL/NHLBI NIH HHS/United States
- UL1 RR024139/RR/NCRR NIH HHS/United States
- UL1 RR024989/RR/NCRR NIH HHS/United States
- RC2 HL102923/HL/NHLBI NIH HHS/United States
- R01 HL113323/HL/NHLBI NIH HHS/United States
- R56 DK062370/DK/NIDDK NIH HHS/United States
- R01 AG046949/AG/NIA NIH HHS/United States
- N01 HC095168/HL/NHLBI NIH HHS/United States
- U54 HG003067/HG/NHGRI NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- RC2 HL102926/HL/NHLBI NIH HHS/United States
- U01 DK085524/DK/NIDDK NIH HHS/United States
- UL1 TR002548/TR/NCATS NIH HHS/United States
- R01 AG057909/AG/NIA NIH HHS/United States
- UM1 DK105554/DK/NIDDK NIH HHS/United States
- U01 DK085545/DK/NIDDK NIH HHS/United States
- U01 HL080295/HL/NHLBI NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- U01 DK057295/DK/NIDDK NIH HHS/United States
- N01 HC095169/HL/NHLBI NIH HHS/United States
- N01 HC085082/HL/NHLBI NIH HHS/United States
- R01 HL092577/HL/NHLBI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- K01 DK107836/DK/NIDDK NIH HHS/United States
- G0900747/MRC_/Medical Research Council/United Kingdom
- UL1 RR025780/RR/NCRR NIH HHS/United States
- U01 HL130114/HL/NHLBI NIH HHS/United States
- K23 DK114551/DK/NIDDK NIH HHS/United States
- R01 DK098032/DK/NIDDK NIH HHS/United States
- N01 HC085083/HL/NHLBI NIH HHS/United States
- M01 RR000069/RR/NCRR NIH HHS/United States
- N01 HC085086/HL/NHLBI NIH HHS/United States
- 212945/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- R01 DK072193/DK/NIDDK NIH HHS/United States
- M01 RR000036/RR/NCRR NIH HHS/United States
- R01 HL086694/HL/NHLBI NIH HHS/United States
- R01 DK114504/DK/NIDDK NIH HHS/United States
- R01 DK110113/DK/NIDDK NIH HHS/United States
- N01 HC095167/HL/NHLBI NIH HHS/United States
- R01 DK107786/DK/NIDDK NIH HHS/United States
- U01 DK061242/DK/NIDDK NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- U54 HG003273/HG/NHGRI NIH HHS/United States
- N01 HC095159/HL/NHLBI NIH HHS/United States
- R01 DK047482/DK/NIDDK NIH HHS/United States
- U01 DK105535/DK/NIDDK NIH HHS/United States
- R01 DK062370/DK/NIDDK NIH HHS/United States
- R01 HL105756/HL/NHLBI NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- RC2 HL102924/HL/NHLBI NIH HHS/United States
- R01 AG008122/AG/NIA NIH HHS/United States
- U01 DK061212/DK/NIDDK NIH HHS/United States
- R01 DK066358/DK/NIDDK NIH HHS/United States
- R01 DK101855/DK/NIDDK NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- N01 HC025195/HL/NHLBI NIH HHS/United States
- R01 AG033193/AG/NIA NIH HHS/United States
- M01 RR014467/RR/NCRR NIH HHS/United States
- N01 HC055222/HL/NHLBI NIH HHS/United States
- F32 DK117558/DK/NIDDK NIH HHS/United States
- K24 DK080140/DK/NIDDK NIH HHS/United States
- U01 DK061230/DK/NIDDK NIH HHS/United States
- RC2 DK088389/DK/NIDDK NIH HHS/United States
- N01 HC085079/HL/NHLBI NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- U01 DK085526/DK/NIDDK NIH HHS/United States
- N01 HC095163/HL/NHLBI NIH HHS/United States
- P01 AG027734/AG/NIA NIH HHS/United States
- U01 DK078616/DK/NIDDK NIH HHS/United States
- U01 DK062370/DK/NIDDK NIH HHS/United States
- U01 DK105554/DK/NIDDK NIH HHS/United States
- P30 AG038072/AG/NIA NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- RC2 HL103010/HL/NHLBI NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- N01 HC095166/HL/NHLBI NIH HHS/United States
- M01 RR000084/RR/NCRR NIH HHS/United States
- N01 HC095162/HL/NHLBI NIH HHS/United States
- MC_PC_13040/MRC_/Medical Research Council/United Kingdom
- R01 AG023629/AG/NIA NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- N01 HC095165/HL/NHLBI NIH HHS/United States
- G0601261/MRC_/Medical Research Council/United Kingdom
- N01 HC095164/HL/NHLBI NIH HHS/United States
- U54 GM115428/GM/NIGMS NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- N01 HC085081/HL/NHLBI NIH HHS/United States
- RC2 HL102925/HL/NHLBI NIH HHS/United States
- P30 DK020595/DK/NIDDK NIH HHS/United States
- M01 RR000125/RR/NCRR NIH HHS/United States
- R01 DK053889/DK/NIDDK NIH HHS/United States
- U01 DK061239/DK/NIDDK NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- N01 HC095160/HL/NHLBI NIH HHS/United States

