Systemic exposure to intracameral vs topical mydriatic agents: in cataract surgery
- PMID: 31118559
- PMCID: PMC6501990
- DOI: 10.2147/OPTH.S189671
Systemic exposure to intracameral vs topical mydriatic agents: in cataract surgery
Abstract
Objective: The objective of this study was to compare systemic exposure to tropicamide/phenylephrine following intracameral or topical administration before cataract surgery.
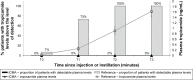
Patients and methods: Mydriatics exposure was calculated in patients randomized to intracameral fixed combination of mydriatics and anesthetic ([ICMA]: tropicamide 0.02%, phenylephrine 0.31%, and lidocaine 1%, N=271) or mydriatic eye drops ([EDs]: tropicamide 0.5% and phenylephrine 10%, N=283). Additional doses were permitted if required. Mydriatic plasma levels were determined by mass spectrometric HPLC in 15 patients per group before and after administration.
Results: Most ICMA patients (73.6%) received a single dose (200 µL) representing an exposure to tropicamide of 0.04 mg and phenylephrine of 0.62 mg. None of these patients received additional mydriatics. In the control group (three administrations), the exposure was 0.45 (11.3-fold higher than ICMA) and 10.2 (16.5-fold higher) mg. When additional ED was used in this group (9.2% of patients), it was 37.5-fold higher for tropicamide (10 drops, 1.5 mg) and 54.8-fold higher for phenylephrine (10 drops, 34 mg) than the recommended ICMA dose. Tropicamide plasma levels were not detectable at any time point in ICMA patients while it was detectable in all ED patients at 12 and 30 minutes. Phenylephrine was detectable in 14.3% of ICMA patients compared to all ED patients at least at one time point. More ED patients experienced a meaningful increase in blood pressure and/or heart rate (11.2% vs 6.0% of ICMA patients; P=0.03).
Conclusion: Systemic exposure to tropicamide/phenylephrine was lower and cardiovascular (CV) effects were less frequent with ICMA. This could be of particular significance in patients at CV risk.
Keywords: cardiovascular safety; cataract surgery; intracameral mydriasis; systemic influence; topical mydriasis.
Conflict of interest statement
Disclosure JG, UP, PR, PJP, PYR, FC, and ML have been engaged as consultants for Laboratoires Théa. FC and ML have received honoraria from Laboratoires Théa. The authors report no other conflicts of interest in this work.
Figures

References
-
- OECD health at a glance: Europe 2014, OECD publishing. 2014. Available from: - DOI
-
- Lundström M, Barry P, Henry Y, Rosen P, Stenevi U. Evidence-based guidelines for cataract surgery: guidelines based on data in the European Registry of quality outcomes for cataract and refractive surgery database. J Cataract Refract Surg. 2012;38(6):1086–1093. - PubMed
-
- Base de Données Publique des medicaments. [Accessed March 22, 2017]. Available from: http://base-donnees-publique.medicaments.gouv.fr/affichageDoc.php?specid....
-
- Levet L, Touzeau O, Scheer S, Borderie V, Laroche L. A study of pupil dilation using the Mydriasert ophthalmic insert. J Fr Ophtalmol. 2004;27(10):1099–1108. French. - PubMed
LinkOut - more resources
Full Text Sources

