Monoclonal antibody therapy of solid tumors: clinical limitations and novel strategies to enhance treatment efficacy
- PMID: 31118560
- PMCID: PMC6503308
- DOI: 10.2147/BTT.S166310
Monoclonal antibody therapy of solid tumors: clinical limitations and novel strategies to enhance treatment efficacy
Abstract
Monoclonal antibodies (mAbs) have become a cornerstone in the therapeutic guidelines of a wide range of solid tumors. The targeted nature of these biotherapeutics has improved treatment outcomes by offering enhanced specificity to reduce severe side effects experienced with conventional chemotherapy. Notwithstanding, poor tumor tissue penetration and the heterogeneous distribution achieved therein are prominent drawbacks that hamper the clinical efficacy of therapeutic antibodies. Failure to deliver efficacious doses throughout the tumor can lead to treatment failure and the development of acquired resistance mechanisms. Comprehending the morphological and physiological characteristics of solid tumors and their microenvironment that affect tumor penetration and distribution is a key requirement to improve clinical outcomes and realize the full potential of monoclonal antibodies in oncology. This review summarizes the essential architectural characteristics of solid tumors that obstruct macromolecule penetration into the targeted tissue following systemic delivery. It further describes mechanisms of resistance elucidated for blockbuster antibodies for which extensive clinical data exists, as a way to illustrate various modes in which cancer cells can overcome the anticancer activity of therapeutic antibodies. Thereafter, it describes novel strategies designed to improve clinical outcomes of mAbs by increasing potency and/or improving tumor delivery; focusing on the recent clinical success and growing clinical pipeline of antibody-drug conjugates, immune checkpoint inhibitors and nanoparticle-based delivery systems.
Keywords: antibody therapy; antibody–drug conjugates; immune checkpoint inhibitors; nanoparticle delivery vehicles; treatment resistance.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures

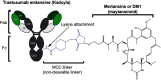

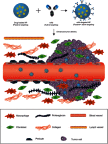
References
-
- Grilo AL, Mantalaris A. The increasingly human and profitable monoclonal antibody market. Trends Biotechnol. 2018;37(1):9–16. - PubMed
-
- U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products. 2018; Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed January 17, 2019
-
- De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753–762. doi:10.1016/S1470-2045(10)70130-3 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

