Dexmedetomidine reduces sevoflurane EC50 for supraglottic airway device insertion in spontaneously breathing morbidly obese patients
- PMID: 31118650
- PMCID: PMC6504637
- DOI: 10.2147/TCRM.S199440
Dexmedetomidine reduces sevoflurane EC50 for supraglottic airway device insertion in spontaneously breathing morbidly obese patients
Abstract
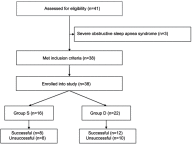
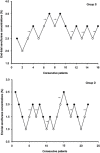
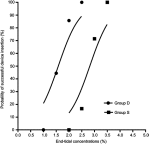
Purpose: This study aimed to assess the effect of intravenous dexmedetomidine (DEX) on sevoflurane EC50 for supraglottic airway device (SAD) insertion in spontaneously breathing morbidly obese patients. Patients and methods: Thirty-eight morbidly obese patients with a body mass index 40-57 kg/m2 who were scheduled for bariatric surgery under general anesthesia requiring tracheal intubation were randomly allocated to two groups receiving the different treatments: group S, saline was given intravenously, and group D, a bolus dose of DEX 1 μg/kg was administered intravenously over 10 mins, followed by intravenous DEX infusion at a rate of 0.5 μg/kg/h. Five percent sevoflurane was initially inhaled for anesthesia induction and then end-tidal expiratory sevoflurane concentration (ETsev) was adjusted to a target value as to the modified Dixon's up-and-down method. Patients' response to SAD insertion was classified as "movement" or "no movement". The average of the midpoints of all crossover points was defined as calculated sevoflurane EC50 for successful SAD insertion. Furthermore, the probit regression analysis was used to determine sevoflurane end-tidal concentrations where 50% (EC50) and 95% (EC95) insertions of SAD were successful. After the observation was completed, flexible bronchoscope-guided intubation was performed through the SAD. Results: The calculated sevoflurane EC50 for successful SAD insertion was significantly lower in group D than in group S (1.75±0.32% vs 2.92±0.26%, p<0.001). By the probit regression analysis, EC50 and EC95 of sevoflurane for successful SAD insertion were 1.59% (95% CI, 1.22-1.90%) and 2.15% (95% CI, 1.86-3.84%) in group D, respectively, and 2.81% (95% CI, 2.35-3.29%) and 3.32% (3.02-6.74%) in group S. Conclusion: When sevoflurane inhalational induction is performed in spontaneous breathing morbidly obese patients, intravenous DEX can reduce sevoflurane EC50 for successful SAD insertion by about 40%. Chinese Clinical Trial Registry: No. ChiCTR1800016868.
Keywords: dexmedetomidine; inhalational induction; obesity; sevoflurane; supraglottic airway device.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
The optimum sevoflurane concentration for supraglottic airway device Blockbuster™ insertion with spontaneous breathing in obese patients: a prospective observational study.BMC Anesthesiol. 2017 Nov 28;17(1):156. doi: 10.1186/s12871-017-0449-5. BMC Anesthesiol. 2017. PMID: 29179689 Free PMC article. Clinical Trial.
-
A feasibility study of jaw thrust as an indicator assessing adequate depth of anesthesia for insertion of supraglottic airway device in morbidly obese patients.Chin Med J (Engl). 2019 Sep 20;132(18):2185-2191. doi: 10.1097/CM9.0000000000000403. Chin Med J (Engl). 2019. PMID: 31425359 Free PMC article.
-
Optimum end-tidal concentration of sevoflurane to facilitate supraglottic airway device insertion with propofol at induction allowing spontaneous respiration in obese patients: A prospective observational study.Medicine (Baltimore). 2017 Nov;96(47):e8902. doi: 10.1097/MD.0000000000008902. Medicine (Baltimore). 2017. PMID: 29382022 Free PMC article.
-
Effects of dexmedetomidine on sevoflurane requirement for 50% excellent tracheal intubation in children: a randomized, double-blind comparison.Paediatr Anaesth. 2014 Sep;24(9):987-93. doi: 10.1111/pan.12430. Epub 2014 May 14. Paediatr Anaesth. 2014. PMID: 24823715 Review.
-
Awake supraglottic airway guided flexible bronchoscopic intubation in patients with anticipated difficult airways: a case series and narrative review.Korean J Anesthesiol. 2019 Dec;72(6):548-557. doi: 10.4097/kja.19318. Epub 2019 Sep 2. Korean J Anesthesiol. 2019. PMID: 31475506 Free PMC article. Review.
Cited by
-
Intraoperative Dexmedetomidine Decreases Postoperative Pain after Gastric Endoscopic Submucosal Dissection: A Prospective Randomized Controlled Trial.J Clin Med. 2023 Feb 24;12(5):1816. doi: 10.3390/jcm12051816. J Clin Med. 2023. PMID: 36902603 Free PMC article.
-
Effect of Intravenous Dexmedetomidine Premedication on Sufentanil Median Effective Concentration During Tracheal Intubation in Obese Patients: A Randomized Controlled Study.Drug Des Devel Ther. 2025 Feb 24;19:1323-1332. doi: 10.2147/DDDT.S491599. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40026333 Free PMC article. Clinical Trial.
-
Addition of Dexmedetomidine to the Anesthesia Regimen Attenuates Pain and Improves Early Recovery After Esophageal Endoscopic Submucosal Dissection: A Randomized Controlled Trial.Drug Des Devel Ther. 2024 Oct 11;18:4551-4562. doi: 10.2147/DDDT.S475749. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39411154 Free PMC article. Clinical Trial.
-
Dexmedetomidine Compared to Remifentanil Infusion as Adjuvant to Sevoflurane Anesthesia during Laparoscopic Sleeve Gastrectomy.Anesth Essays Res. 2019 Oct-Dec;13(4):636-642. doi: 10.4103/aer.AER_126_19. Epub 2019 Dec 16. Anesth Essays Res. 2019. PMID: 32009708 Free PMC article.
References
LinkOut - more resources
Full Text Sources

