Lipids at the Crossroad of α-Synuclein Function and Dysfunction: Biological and Pathological Implications
- PMID: 31118888
- PMCID: PMC6504812
- DOI: 10.3389/fncel.2019.00175
Lipids at the Crossroad of α-Synuclein Function and Dysfunction: Biological and Pathological Implications
Abstract
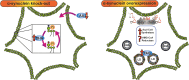
Since its discovery, the study of the biological role of α-synuclein and its pathological implications has been the subject of increasing interest. The propensity to adopt different conformational states governing its aggregation and fibrillation makes this small 14-kDa cytosolic protein one of the main etiologic factors associated with degenerative disorders known as synucleinopathies. The structure, function, and toxicity of α-synuclein and the possibility of different therapeutic approaches to target the protein have been extensively investigated and reviewed. One intriguing characteristic of α-synuclein is the different ways in which it interacts with lipids. Though in-depth studies have been carried out in this field, the information they have produced is puzzling and the precise role of lipids in α-synuclein biology and pathology and vice versa is still largely unknown. Here we provide an overview and discussion of the main findings relating to α-synuclein/lipid interaction and its involvement in the modulation of lipid metabolism and signaling.
Keywords: lipid metabolism; lipid signal transduction; lipids; membrane lipids; α–synuclein.
Figures

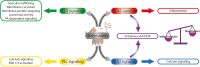
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

