MicroRNA Post-transcriptional Regulation of the NLRP3 Inflammasome in Immunopathologies
- PMID: 31118894
- PMCID: PMC6504709
- DOI: 10.3389/fphar.2019.00451
MicroRNA Post-transcriptional Regulation of the NLRP3 Inflammasome in Immunopathologies
Abstract
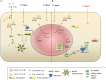
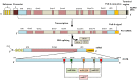
Inflammation has a crucial role in protection against various pathogens. The inflammasome is an intracellular multiprotein signaling complex that is linked to pathogen sensing and initiation of the inflammatory response in physiological and pathological conditions. The most characterized inflammasome is the NLRP3 inflammasome, which is a known sensor of cell stress and is tightly regulated in resting cells. However, altered regulation of the NLRP3 inflammasome is found in several pathological conditions, including autoimmune disease and cancer. NLRP3 expression was shown to be post-transcriptionally regulated and multiple miRNA have been implicated in post-transcriptional regulation of the inflammasome. Therefore, in recent years, miRNA based post-transcriptional control of NLRP3 has become a focus of much research, especially as a potential therapeutic approach. In this review, we provide a summary of the recent investigations on the role of miRNA in the post-transcriptional control of the NLRP3 inflammasome, a key regulator of pro-inflammatory IL-1β and IL-18 cytokine production. Current approaches to targeting the inflammasome product were shown to be an effective treatment for diseases linked to NLRP3 overexpression. Although utilizing NLRP3 targeting miRNAs was shown to be a successful therapeutic approach in several animal models, their therapeutic application in patients remains to be determined.
Keywords: NLRP3; disease; inflammasome; inflammation; microRNA.
Figures

References
-
- Aganna E., Martinon F., Hawkins P. N., Ross J. B., Swan D. C., Booth D. R., et al. (2002). Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 46 2445–2452. 10.1002/art.10509 - DOI - PubMed
-
- Agostini L., Martinon F., Burns K., McDermott M. F., Hawkins P. N., Tschopp J. (2004). NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20 319–325. - PubMed
-
- Alexanderála Pazár M. D. (2010). The Expanded Role of the Inflammasome in Human Disease. Available at: https://www.the-rheumatologist.org/article/the-expanded-role-of-the-infl... (accessed August 01, 2010).
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

