Maternal Restricted- and Over-Feeding During Gestation Result in Distinct Lipid and Amino Acid Metabolite Profiles in the Longissimus Muscle of the Offspring
- PMID: 31118900
- PMCID: PMC6504779
- DOI: 10.3389/fphys.2019.00515
Maternal Restricted- and Over-Feeding During Gestation Result in Distinct Lipid and Amino Acid Metabolite Profiles in the Longissimus Muscle of the Offspring
Abstract
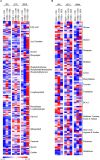
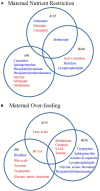
Maternal over- and restricted-feeding during gestation have similar negative consequences for the offspring, including decreased muscularity, increased adiposity, and altered metabolism. Our objective was to determine the effects of poor maternal nutrition during gestation (over- and restricted-feeding) on the offspring muscle metabolite profile. Pregnant ewes (n = 47) were fed 60% (RES), 100% (CON), or 140% (OVER) of NRC requirements starting at day 30.2 ± 0.2 of gestation. Offspring sample collection occurred at days 90 and 135 of gestation, and within 24 h of birth. C2C12 myoblasts were cultured in serum collected from offspring at birth (n = 18; 6 offspring per treatment) for analysis of oxidative and glycolytic capacity. Unbiased metabolite analysis of longissimus muscle samples (n = 72; 8 fetuses per treatment per time point) was performed using mass spectrometry. Data were analyzed by ANOVA for main effects of treatment, time point, and their interaction. Cells cultured in serum from RES offspring exhibited increased proton leak 49% (p = 0.01) compared with CON, but no other variables of mitochondrial respiration or glycolytic function were altered. Mass spectrometry identified 612 metabolites. Principle component analysis identified day of gestation as the primary driver of metabolic change; however, maternal diet also altered the lipid and amino acid profiles in offspring. The abundance of 53 amino acid metabolites and 89 lipid metabolites was altered in RES compared with CON (p ≤ 0.05), including phospholipids, sphingolipids, and ceramides within the lipid metabolism pathway and metabolites involved in glutamate, histidine, and glutathione metabolism. Similarly, abundance of 63 amino acid metabolites and 70 lipid metabolites was altered in OVER compared with CON (p ≤ 0.05). These include metabolites involved in glutamate, histidine, lysine, and tryptophan metabolism and phosphatidylethanolamine, lysophospholipids, and fatty acids involved in lipid metabolism. Further, the amino acid and lipid profiles diverged between RES and OVER, with 69 amino acid and 118 lipid metabolites differing (p ≤ 0.05) between groups. Therefore, maternal diet affects metabolite abundance in offspring longissimus muscle, specifically metabolites involved in lipid and amino metabolism. These changes may impact post-natal skeletal muscle metabolism, possibly altering energy efficiency and long-term health.
Keywords: gestation; maternal diet; metabolism; muscle; offspring.
Figures

Similar articles
-
Poor maternal nutrition during gestation in sheep alters prenatal muscle growth and development in offspring.J Anim Sci. 2020 Jan 1;98(1):skz388. doi: 10.1093/jas/skz388. J Anim Sci. 2020. PMID: 31875422 Free PMC article.
-
Mid- to late-gestational maternal nutrient restriction followed by realimentation alters development and lipid composition of liver and skeletal muscles in ovine fetuses.J Anim Sci. 2021 Dec 1;99(12):skab299. doi: 10.1093/jas/skab299. J Anim Sci. 2021. PMID: 34668541 Free PMC article.
-
Poor maternal diet during gestation alters offspring muscle proteome in sheep.J Anim Sci. 2022 Aug 1;100(8):skac061. doi: 10.1093/jas/skac061. J Anim Sci. 2022. PMID: 35908790 Free PMC article.
-
The Effects of Maternal Nutrient Restriction during Mid to Late Gestation with Realimentation on Fetal Metabolic Profiles in the Liver, Skeletal Muscle, and Blood in Sheep.Metabolites. 2024 Aug 23;14(9):465. doi: 10.3390/metabo14090465. Metabolites. 2024. PMID: 39330472 Free PMC article.
-
Fetal and organ development at gestational days 45, 90, 135 and at birth of lambs exposed to under- or over-nutrition during gestation,.Transl Anim Sci. 2017 Feb 1;1(1):16-25. doi: 10.2527/tas2016.0002. eCollection 2017 Feb. Transl Anim Sci. 2017. PMID: 32704626 Free PMC article.
Cited by
-
Poor maternal nutrition during gestation in sheep alters prenatal muscle growth and development in offspring.J Anim Sci. 2020 Jan 1;98(1):skz388. doi: 10.1093/jas/skz388. J Anim Sci. 2020. PMID: 31875422 Free PMC article.
-
Mid- to late-gestational maternal nutrient restriction followed by realimentation alters development and lipid composition of liver and skeletal muscles in ovine fetuses.J Anim Sci. 2021 Dec 1;99(12):skab299. doi: 10.1093/jas/skab299. J Anim Sci. 2021. PMID: 34668541 Free PMC article.
-
Maternal Nutrition During Gestation Alters Histochemical Properties, and mRNA and microRNA Expression in Adipose Tissue of Wagyu Fetuses.Front Endocrinol (Lausanne). 2022 Feb 1;12:797680. doi: 10.3389/fendo.2021.797680. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35178028 Free PMC article.
-
Poor maternal diet during gestation alters offspring muscle proteome in sheep.J Anim Sci. 2022 Aug 1;100(8):skac061. doi: 10.1093/jas/skac061. J Anim Sci. 2022. PMID: 35908790 Free PMC article.
-
The Effects of Maternal Nutrient Restriction during Mid to Late Gestation with Realimentation on Fetal Metabolic Profiles in the Liver, Skeletal Muscle, and Blood in Sheep.Metabolites. 2024 Aug 23;14(9):465. doi: 10.3390/metabo14090465. Metabolites. 2024. PMID: 39330472 Free PMC article.
References
-
- Baron A. D., Brechtel G., Wallace P., Edelman S. V. (1988). Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am. J. Physiol. 255 E769–E774. - PubMed
LinkOut - more resources
Full Text Sources

