Adverse Events Associated with Ethical Kampo Formulations: Analysis of the Domestic Adverse-Event Data Reports of the Ministry of Health, Labor, and Welfare in Japan
- PMID: 31118950
- PMCID: PMC6500660
- DOI: 10.1155/2019/1643804
Adverse Events Associated with Ethical Kampo Formulations: Analysis of the Domestic Adverse-Event Data Reports of the Ministry of Health, Labor, and Welfare in Japan
Abstract
Objectives: Traditional Japanese Kampo medicines have been integrated into the Japanese national health-care system. In Japan, the Ministry of Health, Labor, and Welfare's website discloses adverse drug-event data that have been obtained from medical personnel reports investigated by the Pharmaceutical and Medical Devices Agency. Using these data, we investigated adverse events associated with ethical Kampo formulations.
Methods: Reports of adverse events associated with ethical Kampo formulations from the domestic adverse-event data were obtained from July 30, 2003, to March 31, 2018. Adverse events were then categorized, and the relationships between categories of adverse events and crude drugs were analyzed.
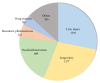
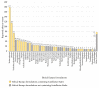
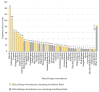
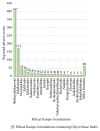
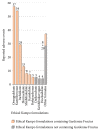
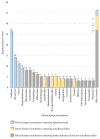
Results: There were 4,232 reported adverse events associated with ethical Kampo formulations. The numbers of events by category were as follows: events related to liver injury, 1,193; lung injury, 1,177; pseudoaldosteronism, 889; mesenteric phlebosclerosis, 223; drug eruption, 185; and others, 565. Among events related to both liver injury and lung injury, approximately 70% were suspected to be induced by Kampo formulations containing Scutellariae Radix. The pseudoaldosteronism-related events, which are induced by Glycyrrhizae Radix, included several events related to muscle injury, heart failure, and arrhythmia. Events related to mesenteric phlebosclerosis, believed to be induced by long-term use of Kampo formulas containing Gardeniae Fructus, increased remarkably during the study period. Among the events related to drug eruption, approximately 35% were suspected to be induced by Kampo formulations containing Ephedrae Herba.
Conclusion: Kampo medicines may cause various adverse events. The present results provide valuable information regarding adverse events associated with Kampo medicines from the viewpoint of patient safety.
Figures
References
-
- Shimada Y., Fujimoto M., Nogami T., et al. Patient safety incident reports related to traditional Japanese Kampo medicines: medication errors and adverse drug events in a university hospital for a ten-year period. BMC Complementary and Alternative Medicine. 2017;17(1, article no. 547) doi: 10.1186/s12906-017-2051-2. - DOI - PMC - PubMed
-
- Actual Condition Survey on Prescription of Kampo Formulas 2011 . Tokyo, Japan: Japan Kampo Medicines Manufacturers Association; 2011. (Jpn).
LinkOut - more resources
Full Text Sources

