Reactive capillary hemangiomas: a novel dermatologic toxicity following anti-PD-1 treatment with SHR-1210
- PMID: 31119058
- PMCID: PMC6528453
- DOI: 10.20892/j.issn.2095-3941.2018.0172
Reactive capillary hemangiomas: a novel dermatologic toxicity following anti-PD-1 treatment with SHR-1210
Abstract
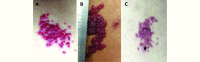
Objective: SHR-1210 is a new and promising anti-PD-1 agent for solid tumors. During the phase I study of SHR-1210, we encountered a novel but prevalent immune-related dermatologic toxicity: reactive capillary hemangiomas (RCHs). Thus we tried to summarize the features of RCHs and estimate their relationship with tumor response.
Methods: This prospective observational study systematically enrolled 98 patients with advanced solid tumors from April 27th, 2016 to June 8th, 2017 in the context of the phase I clinical study of SHR-1210. This report focused on the skin toxicities. Patients underwent entire skin inspection every two weeks while taking medication. The clinical course of RCHs was recorded and their association with tumor response was estimated. The data cut-off date was November 15th, 2017.
Results: After a median follow-up of 242 (range, 29-567) days, RCHs were observed in 85.7% (84/98) of patients on cutaneous/mucosal surfaces; 84.5% (71/84) of the RCHs were evaluated as grade 1 adverse events. No grade 3 or 4 RCHs were observed. The time of onset of RCHs was dose dependent and shortest in the 400 mg-dose cohort (P < 0.001). Spontaneous and complete regression of RCHs was observed both during and after treatment. The objective response rate of tumors for patients with RCHs was 28.9% (24/83). However, no responders were observed among the patients without RCHs.
Conclusions: RCHs were prevalent but manageable during treatment with SHR-1210. It might add to the expanding literature regarding immune-related dermatologic adverse events.
Keywords: Reactive capillary hemangiomas; SHR-1210; anti-tumor efficacy; skin toxicity.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous
