Molecular epidemiology of coagulase-negative Staphylococcus species isolated at different lactation stages from dairy cattle in the United States
- PMID: 31119068
- PMCID: PMC6507897
- DOI: 10.7717/peerj.6749
Molecular epidemiology of coagulase-negative Staphylococcus species isolated at different lactation stages from dairy cattle in the United States
Abstract
Background: Coagulase negative Staphylococcus (CNS) species are currently the most prevalent intra-mammary pathogens causing subclinical mastitis and occasional clinical mastitis or persistent infection in lactating dairy cattle. More than 10 CNS species have been identified, but they are generally managed as one group on most dairies in the United States. However, improved management decisions and treatment outcomes may be achieved with better understanding of the prevalent species, pathogenicity and strain diversity within and across dairies.
Methodology: A total of 604 CNS isolates were cultured from milk samples collected during a dry-cow treatment clinical trial conducted on 6 dairy herds in 4 states in the US. All the study cows were randomized to receive 1 of the 3 different intra-mammary antimicrobial infusions (Quatermaster, Spectramast DC or ToMorrow Dry Cow) at dry-off. Milk samples were collected at dry-off, calving (0-6 days in milk, DIM), post-calving (7-13 DIM) and at mastitis events within the first 100 DIM. The CNS isolates were identified to species level by partial sequencing of the rpoβ gene, and genetic relatedness within species was investigated by phylogenetic analysis of the pulse-field gel electrophoresis profiles of the isolates.
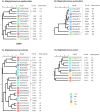
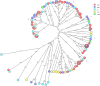
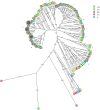
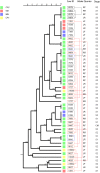
Results: The major CNS species identified were S. chromogenes (48.3%), S. haemolyticus (17.9%), S. simulans and S. epidermidis (each at 6.5%). Other CNS species identified at lower frequencies included S. hominis, S. auricularis, S. sciuri, S. spp KS-SP, S. capitis, S. cohnii, S. warneri, S. pasteuri, S. xylosus, S. hyicus, S. equorum, S. microti, S. rostri, S. gallinarum, S. saprophyticus and S. succinus. Phylogenetic analyses of the major species types demonstrated an association between genetic relatedness and epidemiological distributions of S. chromogenes, S. simulans, S. haemolyticus and S. auricularis. Additionally, identical strains of S. chromogenes and S. simulans were isolated from the same udder quarter of several cows at consecutive sample stages. The rest of the minor species had no deducible genetic-epidemiological link.
Discussion: The observed association between genetic and epidemiological distributions indicated animal-adapted nature of four CNS species, suggesting possible host-adapted and environmental transmission of these species. Multi-stage isolation of the same udder quarter strain was evidence for chronic intra-mammary infection.
Conclusion: The different CNS species and strains circulating on US dairy herds were genetically diverse. Four species identified were likely udder-adapted pathogens, 2 of which caused persistent infection. Our findings are important in guiding the design of effective mastitis control strategies.
Keywords: Coagulase negative Staphylococcus (CNS); Epidemiology; Genetic relatedness; Mastitis pathogens; Phylogeny; Pulse-field gel electrophoresis (PFGE).
Conflict of interest statement
The authors declare there are no competing interests.
Figures

References
-
- Arruda A, Godden S, Rapnicki P, Gorden P, Timms L, Aly SS, Lehenbauer TW, Champagne J. Randomized noninferiority clinical trial evaluating 3 commercial dry cow mastitis preparations: I. Quarter-level outcomes. Journal of Dairy Science. 2013;96(7):4419–4435. doi: 10.3168/jds.2012-6461. - DOI - PubMed
-
- Björk S, Båge R, Kanyima BM, André S, Nassuna-Musoke MG, Owiny DO, Persson Y. Characterization of coagulase negative Staphylococci from cases of subclinical mastitis in dairy cattle in Kampala, Uganda. Irish Veterinary Journal. 2014;67(1) doi: 10.1186/2046-0481-67-12. Article 12. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

