The Aluminum-Ion Battery: A Sustainable and Seminal Concept?
- PMID: 31119122
- PMCID: PMC6504778
- DOI: 10.3389/fchem.2019.00268
The Aluminum-Ion Battery: A Sustainable and Seminal Concept?
Abstract
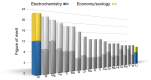
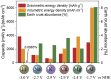
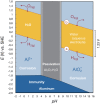
The expansion of renewable energy and the growing number of electric vehicles and mobile devices are demanding improved and low-cost electrochemical energy storage. In order to meet the future needs for energy storage, novel material systems with high energy densities, readily available raw materials, and safety are required. Currently, lithium and lead mainly dominate the battery market, but apart from cobalt and phosphorous, lithium may show substantial supply challenges prospectively, as well. Therefore, the search for new chemistries will become increasingly important in the future, to diversify battery technologies. But which materials seem promising? Using a selection algorithm for the evaluation of suitable materials, the concept of a rechargeable, high-valent all-solid-state aluminum-ion battery appears promising, in which metallic aluminum is used as the negative electrode. On the one hand, this offers the advantage of a volumetric capacity four times higher (theoretically) compared to lithium analog. On the other hand, aluminum is the most abundant metal in the earth's crust. There is a mature industry and recycling infrastructure, making aluminum very cost efficient. This would make the aluminum-ion battery an important contribution to the energy transition process, which has already started globally. So far, it has not been possible to exploit this technological potential, as suitable positive electrodes and electrolyte materials are still lacking. The discovery of inorganic materials with high aluminum-ion mobility-usable as solid electrolytes or intercalation electrodes-is an innovative and required leap forward in the field of rechargeable high-valent ion batteries. In this review article, the constraints for a sustainable and seminal battery chemistry are described, and we present an assessment of the chemical elements in terms of negative electrodes, comprehensively motivate utilizing aluminum, categorize the aluminum battery field, critically review the existing positive electrodes and solid electrolytes, present a promising path for the accelerated development of novel materials and address problems of scientific communication in this field.
Keywords: aluminum-ion battery; cathode; electrolyte; post-lithium; resources.
Figures



References
-
- Adams S., Rao R. P. (2011). High power lithium ion battery materials by computational design. Phys. Status Solidi A 208, 1746–1753. 10.1002/pssa.201001116 - DOI
-
- Ahmed E., Ruck M. (2011). Homo- and heteroatomic polycations of groups 15 and 16. Recent advances in synthesis and isolation using room temperature ionic liquids. Coord. Chem. Rev. 225, 2892–2903. 10.1016/j.ccr.2011.06.011 - DOI
-
- Anurova N. A., Blatov V. A., Ilyushin G. D., Blatova O. A., Ivanov-Schitz A. K., Dem'yanet L. N. (2008). Migration maps of Li+ cations in oxygen-containing compounds. Solid State Ionics 179, 2248–2254. 10.1016/j.ssi.2008.08.001 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

