Autoimmune Calcium Channelopathies and Cardiac Electrical Abnormalities
- PMID: 31119135
- PMCID: PMC6507622
- DOI: 10.3389/fcvm.2019.00054
Autoimmune Calcium Channelopathies and Cardiac Electrical Abnormalities
Abstract
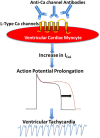
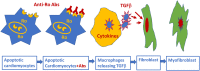
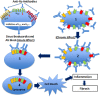
Patients with autoimmune diseases are at increased risk for developing cardiovascular diseases, and abnormal electrocardiographic findings are common. Voltage-gated calcium channels play a major role in the cardiovascular system and regulate cardiac excitability and contractility. Particularly, by virtue of their localization and expression in the heart, calcium channels modulate pace making at the sinus node, conduction at the atrioventricular node and cardiac repolarization in the working myocardium. Consequently, emerging evidence suggests that calcium channels are targets to autoantibodies in autoimmune diseases. Autoimmune-associated cardiac calcium channelopathies have been recognized in both sinus node dysfunction atrioventricular block in patients positive for anti-Ro/La antibodies, and ventricular arrhythmias in patients with dilated cardiomyopathy. In this review, we discuss mechanisms of autoimmune-associated calcium channelopathies and their relationship with the development of cardiac electrical abnormalities.
Keywords: autoantibodies; autoimmune; calcium channel; cardiac electrical abnormalities; channelopathy.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

