Muscular dystrophy with arrhythmia caused by loss-of-function mutations in BVES
- PMID: 31119192
- PMCID: PMC6501641
- DOI: 10.1212/NXG.0000000000000321
Muscular dystrophy with arrhythmia caused by loss-of-function mutations in BVES
Abstract
Objective: To study the genetic and phenotypic spectrum of patients harboring recessive mutations in BVES.
Methods: We performed whole-exome sequencing in a multicenter cohort of 1929 patients with a suspected hereditary myopathy, showing unexplained limb-girdle muscular weakness and/or elevated creatine kinase levels. Immunohistochemistry and mRNA experiments on patients' skeletal muscle tissue were performed to study the pathogenicity of identified loss-of-function (LOF) variants in BVES.
Results: We identified 4 individuals from 3 families harboring homozygous LOF variants in BVES, the gene that encodes for Popeye domain containing protein 1 (POPDC1). Patients showed skeletal muscle involvement and cardiac conduction abnormalities of varying nature and severity, but all exhibited at least subclinical signs of both skeletal muscle and cardiac disease. All identified mutations lead to a partial or complete loss of function of BVES through nonsense-mediated decay or through functional changes to the POPDC1 protein.
Conclusions: We report the identification of homozygous LOF mutations in BVES, causal in a young adult-onset myopathy with concomitant cardiac conduction disorders in the absence of structural heart disease. These findings underline the role of POPDC1, and by extension, other members of this protein family, in striated muscle physiology and disease. This disorder appears to have a low prevalence, although it is probably underdiagnosed because of its striking phenotypic variability and often subtle yet clinically relevant manifestations, particularly concerning the cardiac conduction abnormalities.
Figures


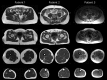
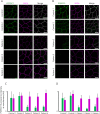
References
-
- Vissing J. Limb girdle muscular dystrophies: classification, clinical spectrum and emerging therapies. Curr Opin Neurol 2016;29:635–641. - PubMed
-
- Silvestri NJ, Ismail H, Zimetbaum P, Raynor EM. Cardiac involvement in the muscular dystrophies. Muscle Nerve 2018;57:707–715. - PubMed
-
- Narayanaswami P, Weiss M, Selcen D, et al. . Evidence-based guideline summary: diagnosis and treatment of limb-girdle and distal dystrophies: report of the guideline development subcommittee of the American Academy of Neurology and the practice issues review panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology 2014;83:1453–1463. - PMC - PubMed
-
- Rezazadeh S, Duff HJ. Genetic determinants of hereditary bradyarrhythmias: a contemporary review of a diverse group of disorders. Can J Cardiol 2017;33:758–767. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
