Omics Technologies to Understand Activation of a Biosynthetic Gene Cluster in Micromonospora sp. WMMB235: Deciphering Keyicin Biosynthesis
- PMID: 31120241
- PMCID: PMC6591704
- DOI: 10.1021/acschembio.9b00223
Omics Technologies to Understand Activation of a Biosynthetic Gene Cluster in Micromonospora sp. WMMB235: Deciphering Keyicin Biosynthesis
Abstract
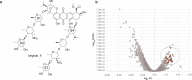
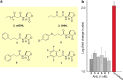
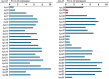
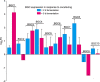
DNA sequencing of a large collection of bacterial genomes reveals a wealth of orphan biosynthetic gene clusters (BGCs) with no identifiable products. BGC silencing, for those orphan clusters that are truly silent, rather than those whose products have simply evaded detection and cluster correlation, is postulated to result from transcriptional inactivation of these clusters under standard laboratory conditions. Here, we employ a multi-omics approach to demonstrate how interspecies interactions modulate the keyicin producing kyc cluster at the transcriptome level in cocultures of kyc-bearing Micromonospora sp. and a Rhodococcus sp. We further correlate coculture dependent changes in keyicin production to changes in transcriptomic and proteomic profiles and show that these changes are attributable to small molecule signaling consistent with a quorum sensing pathway. In piecing together the various elements underlying keyicin production in coculture, this study highlights how omics technologies can expedite future efforts to understand and exploit silent BGCs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

