NMR experiments redefine the hemoglobin binding properties of bacterial NEAr-iron Transporter domains
- PMID: 31120610
- PMCID: PMC6635774
- DOI: 10.1002/pro.3662
NMR experiments redefine the hemoglobin binding properties of bacterial NEAr-iron Transporter domains
Abstract
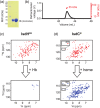
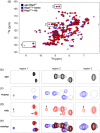
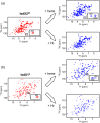
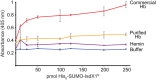
Iron is a versatile metal cofactor that is used in a wide range of essential cellular processes. During infections, many bacterial pathogens acquire iron from human hemoglobin (Hb), which contains the majority of the body's total iron content in the form of heme (iron protoporphyrin IX). Clinically important Gram-positive bacterial pathogens scavenge heme using an array of secreted and cell-wall-associated receptors that contain NEAr-iron Transporter (NEAT) domains. Experimentally defining the Hb binding properties of NEAT domains has been challenging, limiting our understanding of their function in heme uptake. Here we show that solution-state NMR spectroscopy is a powerful tool to define the Hb binding properties of NEAT domains. The utility of this method is demonstrated using the NEAT domains from Bacillus anthracis and Listeria monocytogenes. Our results are compatible with the existence of at least two types of NEAT domains that are capable of interacting with either Hb or heme. These binding properties can be predicted from their primary sequences, with Hb- and heme-binding NEAT domains being distinguished by the presence of (F/Y)YH(Y/F) and S/YXXXY motifs, respectively. The results of this work should enable the functions of a wide range of NEAT domain containing proteins in pathogenic bacteria to be reliably predicted.
Keywords: NEAT domain; NMR spectroscopy; bacteria; heme; hemoglobin; pathogen.
© 2019 The Protein Society.
Figures

Similar articles
-
NEAr Transporter (NEAT) Domains: Unique Surface Displayed Heme Chaperones That Enable Gram-Positive Bacteria to Capture Heme-Iron From Hemoglobin.Front Microbiol. 2021 Jan 6;11:607679. doi: 10.3389/fmicb.2020.607679. eCollection 2020. Front Microbiol. 2021. PMID: 33488548 Free PMC article. Review.
-
Differential function of lip residues in the mechanism and biology of an anthrax hemophore.PLoS Pathog. 2012;8(3):e1002559. doi: 10.1371/journal.ppat.1002559. Epub 2012 Mar 8. PLoS Pathog. 2012. PMID: 22412371 Free PMC article.
-
The five near-iron transporter (NEAT) domain anthrax hemophore, IsdX2, scavenges heme from hemoglobin and transfers heme to the surface protein IsdC.J Biol Chem. 2011 Sep 23;286(38):33652-60. doi: 10.1074/jbc.M111.241687. Epub 2011 Aug 1. J Biol Chem. 2011. PMID: 21808055 Free PMC article.
-
The near-iron transporter (NEAT) domains of the anthrax hemophore IsdX2 require a critical glutamine to extract heme from methemoglobin.J Biol Chem. 2013 Mar 22;288(12):8479-8490. doi: 10.1074/jbc.M112.430009. Epub 2013 Jan 30. J Biol Chem. 2013. PMID: 23364793 Free PMC article.
-
Structural biology of heme binding in the Staphylococcus aureus Isd system.J Inorg Biochem. 2010 Mar;104(3):341-8. doi: 10.1016/j.jinorgbio.2009.09.012. Epub 2009 Sep 26. J Inorg Biochem. 2010. PMID: 19853304 Review.
Cited by
-
Pirates of the haemoglobin.Microb Cell. 2022 Feb 18;9(4):84-102. doi: 10.15698/mic2022.04.775. eCollection 2022 Apr 4. Microb Cell. 2022. PMID: 35434122 Free PMC article. Review.
-
Molecular basis of hemoglobin binding and heme removal in Corynebacterium diphtheriae.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2411833122. doi: 10.1073/pnas.2411833122. Epub 2024 Dec 31. Proc Natl Acad Sci U S A. 2025. PMID: 39739808 Free PMC article.
-
The Shr receptor from Streptococcus pyogenes uses a cap and release mechanism to acquire heme-iron from human hemoglobin.Proc Natl Acad Sci U S A. 2023 Jan 31;120(5):e2211939120. doi: 10.1073/pnas.2211939120. Epub 2023 Jan 24. Proc Natl Acad Sci U S A. 2023. PMID: 36693107 Free PMC article.
-
NEAr Transporter (NEAT) Domains: Unique Surface Displayed Heme Chaperones That Enable Gram-Positive Bacteria to Capture Heme-Iron From Hemoglobin.Front Microbiol. 2021 Jan 6;11:607679. doi: 10.3389/fmicb.2020.607679. eCollection 2020. Front Microbiol. 2021. PMID: 33488548 Free PMC article. Review.
-
The Staphylococcus aureus IsdH Receptor Forms a Dynamic Complex with Human Hemoglobin that Triggers Heme Release via Two Distinct Hot Spots.J Mol Biol. 2020 Feb 14;432(4):1064-1082. doi: 10.1016/j.jmb.2019.12.023. Epub 2019 Dec 24. J Mol Biol. 2020. PMID: 31881209 Free PMC article.
References
-
- Nobles CL, Maresso AW (2011) The theft of host heme by Gram‐positive pathogenic bacteria. Metallomics 3:788–796. - PubMed
-
- Sheldon JR, Heinrichs DE (2015) Recent developments in understanding the iron acquisition strategies of gram positive pathogens. FEMS Microbiol Rev 39:592–630. - PubMed
-
- Hare SA (2017) Diverse structural approaches to haem appropriation by pathogenic bacteria. Biochim Biophys Acta Proteins Proteomics 1865:422–433. - PubMed

