Combining Nanomedicine and Immunotherapy
- PMID: 31120725
- PMCID: PMC7115879
- DOI: 10.1021/acs.accounts.9b00148
Combining Nanomedicine and Immunotherapy
Abstract
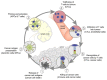
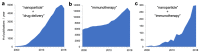
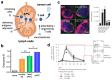
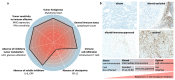
Nanomedicine holds significant potential to improve the efficacy of cancer immunotherapy. Thus far, nanomedicines, i.e., 1-100(0) nm sized drug delivery systems, have been primarily used to improve the balance between the efficacy and toxicity of conjugated or entrapped chemotherapeutic drugs. The clinical performance of cancer nanomedicines has been somewhat disappointing, which is arguably mostly due to the lack of tools and technologies for patient stratification. Conversely, the clinical progress made with immunotherapy has been spectacular, achieving complete cures and inducing long-term survival in advanced-stage patients. Unfortunately, however, immunotherapy only works well in relatively small subsets of patients. Increasing amounts of preclinical and clinical data demonstrate that combining nanomedicine with immunotherapy can boost therapeutic outcomes, by turning "cold" nonimmunoresponsive tumors and metastases into "hot" immunoresponsive lesions. Nano-immunotherapy can be realized via three different approaches, in which nanomedicines are used (1) to target cancer cells, (2) to target the tumor immune microenvironment, and (3) to target the peripheral immune system. When targeting cancer cells, nanomedicines typically aim to induce immunogenic cell death, thereby triggering the release of tumor antigens and danger-associated molecular patterns, such as calreticulin translocation, high mobility group box 1 protein and adenosine triphosphate. The latter serve as adjuvants to alert antigen-presenting cells to take up, process and present the former, thereby promoting the generation of CD8+ cytotoxic T cells. Nanomedicines targeting the tumor immune microenvironment potentiate cancer immunotherapy by inhibiting immunosuppressive cells, such as M2-like tumor-associated macrophages, as well as by reducing the expression of immunosuppressive molecules, such as transforming growth factor beta. In addition, nanomedicines can be employed to promote the activity of antigen-presenting cells and cytotoxic T cells in the tumor immune microenvironment. Nanomedicines targeting the peripheral immune system aim to enhance antigen presentation and cytotoxic T cell production in secondary lymphoid organs, such as lymph nodes and spleen, as well as to engineer and strengthen peripheral effector immune cell populations, thereby promoting anticancer immunity. While the majority of immunomodulatory nanomedicines are in preclinical development, exciting results have already been reported in initial clinical trials. To ensure efficient translation of nano-immunotherapy constructs and concepts, we have to consider biomarkers in their clinical development, to make sure that the right nanomedicine formulation is combined with the right immunotherapy in the right patient. In this context, we have to learn from currently ongoing efforts in nano-biomarker identification as well as from partially already established immuno-biomarker initiatives, such as the Immunoscore and the cancer immunogram. Together, these protocols will help to capture the nano-immuno status in individual patients, enabling the identification and use of individualized and improved nanomedicine-based treatments to boost the performance of cancer immunotherapy.
Figures


Similar articles
-
Tumor-Targeted Nanomedicine for Immunotherapy.Acc Chem Res. 2020 Dec 15;53(12):2765-2776. doi: 10.1021/acs.accounts.0c00518. Epub 2020 Nov 8. Acc Chem Res. 2020. PMID: 33161717 Review.
-
Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy.Theranostics. 2022 Jan 1;12(1):434-458. doi: 10.7150/thno.67300. eCollection 2022. Theranostics. 2022. PMID: 34987658 Free PMC article. Review.
-
Cancer nanomedicine meets immunotherapy: opportunities and challenges.Acta Pharmacol Sin. 2020 Jul;41(7):954-958. doi: 10.1038/s41401-020-0448-9. Epub 2020 Jun 17. Acta Pharmacol Sin. 2020. PMID: 32555445 Free PMC article. Review.
-
Smart cancer nanomedicine.Nat Nanotechnol. 2019 Nov;14(11):1007-1017. doi: 10.1038/s41565-019-0567-y. Epub 2019 Nov 6. Nat Nanotechnol. 2019. PMID: 31695150 Free PMC article.
-
Nanobodies targeting the tumor microenvironment and their formulation as nanomedicines.Mol Cancer. 2025 Mar 4;24(1):65. doi: 10.1186/s12943-025-02270-5. Mol Cancer. 2025. PMID: 40033293 Free PMC article. Review.
Cited by
-
Nanomaterial-Based Drug Delivery Systems: A New Weapon for Cancer Immunotherapy.Int J Nanomedicine. 2022 Oct 3;17:4677-4696. doi: 10.2147/IJN.S376216. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36211025 Free PMC article. Review.
-
Penetrable Nanoplatform for "Cold" Tumor Immune Microenvironment Reeducation.Adv Sci (Weinh). 2020 Jul 29;7(17):2000411. doi: 10.1002/advs.202000411. eCollection 2020 Sep. Adv Sci (Weinh). 2020. PMID: 32995118 Free PMC article.
-
Ferroptosis and Its Potential Role in Glioma: From Molecular Mechanisms to Therapeutic Opportunities.Antioxidants (Basel). 2022 Oct 28;11(11):2123. doi: 10.3390/antiox11112123. Antioxidants (Basel). 2022. PMID: 36358495 Free PMC article. Review.
-
A Snapshot of Photoresponsive Liposomes in Cancer Chemotherapy and Immunotherapy: Opportunities and Challenges.ACS Omega. 2023 Nov 14;8(47):44424-44436. doi: 10.1021/acsomega.3c04134. eCollection 2023 Nov 28. ACS Omega. 2023. PMID: 38046305 Free PMC article. Review.
-
Second near-infrared photothermal-amplified immunotherapy using photoactivatable composite nanostimulators.J Nanobiotechnology. 2021 Dec 20;19(1):433. doi: 10.1186/s12951-021-01197-5. J Nanobiotechnology. 2021. PMID: 34930269 Free PMC article.
References
-
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. - PubMed
-
- Stoeva SI, Lee JS, Smith JE, Rosen ST, Mirkin CA. Multiplexed detection of protein cancer markers with biobarcoded nanoparticle probes. J Am Chem Soc. 2006;128:8378–8379. - PubMed
-
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. - PubMed
-
- Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvasc Res. 1986;31:288–305. - PubMed
-
- Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161:175–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

