Characterization of a cold-active, detergent-stable metallopeptidase purified from Bacillus sp. S1DI 10 using Response Surface Methodology
- PMID: 31120932
- PMCID: PMC6532869
- DOI: 10.1371/journal.pone.0216990
Characterization of a cold-active, detergent-stable metallopeptidase purified from Bacillus sp. S1DI 10 using Response Surface Methodology
Abstract
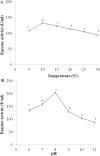
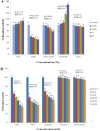
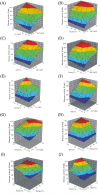
The colder regions of Earth are inhabited by cold-adapted microorganisms designated as psychrophiles that are known to produce cold-active enzymes, such as peptidases, chaperones, lipases, cellulases, and phosphatases. These types of enzymes are a major part of the market of industrial enzymes. Bacteria isolated from water samples collected from the Chamba region in the Himalayas were screened for peptidase production using skim milk agar plates. Among the peptidase-producing bacteria isolated, 20% of the isolates exhibited fast growth and maximum zones of clearance, and thus, were used for further studies. The 16S rDNA sequence analysis of isolate S1DI 10 identified it as a Bacillus sp. The peptidase was cloned in pET28a vector and expressed in Escherichia coli BL21(DE3) and the His-tagged recombinant protein was purified using Ni-NTA column. The purified peptidase of SIDI 10 was found to be an alkaline, cold-active peptidase with optimal enzyme activity at 10°C and pH 8. An approach of one variable at a time was used to further study the effect of various metal ions, organic solvents and detergents on the peptidase enzyme. The peptidase activity was enhanced in the presence of Fe2+ and Mn2+ (metal ions), hexane (organic solvent), SDS- sodium dodecyl sulfate (anionic detergent) and Tween 80 (nonionic detergent). Response surface methodology (RSM) was used to determine the cumulative effect of these five variables. A 25 full factorial central composite design was applied for the five independent variables to determine the optimal combinations of these constituents at the maximum peptidase activity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Purification and characterization of a thermo- and organic solvent-tolerant alkaline protease from Bacillus sp. JER02.Prep Biochem Biotechnol. 2015;45(2):128-43. doi: 10.1080/10826068.2014.907176. Prep Biochem Biotechnol. 2015. PMID: 24845261
-
Detergent-compatible, organic solvent-tolerant alkaline protease from Bacillus circulans MTCC 7942: Purification and characterization.Prep Biochem Biotechnol. 2016;46(1):56-64. doi: 10.1080/10826068.2014.979205. Prep Biochem Biotechnol. 2016. PMID: 25356983
-
Cloning, purification, and characterization of a cold-adapted esterase produced by Psychrobacter cryohalolentis K5T from Siberian cryopeg.FEMS Microbiol Ecol. 2012 Nov;82(2):367-75. doi: 10.1111/j.1574-6941.2012.01385.x. Epub 2012 Apr 30. FEMS Microbiol Ecol. 2012. PMID: 22486752
-
Detergent-compatible bacterial cellulases.J Basic Microbiol. 2019 Feb;59(2):134-147. doi: 10.1002/jobm.201800436. Epub 2018 Nov 13. J Basic Microbiol. 2019. PMID: 30421443 Review.
-
Current prospective in using cold-active enzymes as eco-friendly detergent additive.Appl Microbiol Biotechnol. 2020 Apr;104(7):2871-2882. doi: 10.1007/s00253-020-10429-x. Epub 2020 Feb 10. Appl Microbiol Biotechnol. 2020. PMID: 32037467 Review.
Cited by
-
Cold-Adapted Proteases: An Efficient and Energy-Saving Biocatalyst.Int J Mol Sci. 2023 May 10;24(10):8532. doi: 10.3390/ijms24108532. Int J Mol Sci. 2023. PMID: 37239878 Free PMC article. Review.
References
-
- Margesin R., Schinner F., Marx J.C., Gerday C. (2008). Psychrophiles: from Biodiversity to Biotechnology. Springer, Berlin, Heidelberg: 211–224.
-
- Deming J.W. (2002). Psychrophiles and polar regions. Curr. Opin. Biotechnol. 5, 301–309. - PubMed
-
- Godfrey T., West S. (1996). Introduction to industrial enzymology In Industrial Enzymology, 2nd ed, Godfrey W. Ed.; Macmillan Press: London, UK: 1–8.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

