Endothelial Hypoxia-Inducible Factor-1α Is Required for Vascular Repair and Resolution of Inflammatory Lung Injury through Forkhead Box Protein M1
- PMID: 31121134
- PMCID: PMC6680254
- DOI: 10.1016/j.ajpath.2019.04.014
Endothelial Hypoxia-Inducible Factor-1α Is Required for Vascular Repair and Resolution of Inflammatory Lung Injury through Forkhead Box Protein M1
Abstract
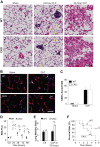
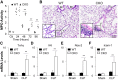
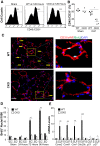
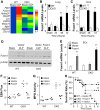
Endothelial barrier dysfunction is a central factor in the pathogenesis of persistent lung inflammation and protein-rich edema formation, the hallmarks of acute respiratory distress syndrome. However, little is known about the molecular mechanisms that are responsible for vascular repair and resolution of inflammatory injury after sepsis challenge. Herein, we show that hypoxia-inducible factor-1α (HIF-1α), expressed in endothelial cells (ECs), is the critical transcriptional factor mediating vascular repair and resolution of inflammatory lung injury. After sepsis challenge, HIF-1α but not HIF-2α expression was rapidly induced in lung vascular ECs, and mice with EC-restricted disruption of Hif1α (Hif1af/f/Tie2Cre+) exhibited defective vascular repair, persistent inflammation, and increased mortality in contrast with the wild-type littermates after polymicrobial sepsis or endotoxemia challenge. Hif1af/f/Tie2Cre+ lungs exhibited marked decrease of EC proliferation during recovery after sepsis challenge, which was associated with inhibited expression of forkhead box protein M1 (Foxm1), a reparative transcription factor. Therapeutic restoration of endothelial Foxm1 expression, via liposomal delivery of Foxm1 plasmid DNA to Hif1af/f/Tie2Cre+ mice, resulted in reactivation of the vascular repair program and improved survival. Together, our studies, for the first time, delineate the essential role of endothelial HIF-1α in driving the vascular repair program. Thus, therapeutic activation of HIF-1α-dependent vascular repair may represent a novel and effective therapy to treat inflammatory vascular diseases, such as sepsis and acute respiratory distress syndrome.
Copyright © 2019 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Rabeprazole Promotes Vascular Repair and Resolution of Sepsis-Induced Inflammatory Lung Injury through HIF-1α.Cells. 2022 Apr 22;11(9):1425. doi: 10.3390/cells11091425. Cells. 2022. PMID: 35563731 Free PMC article.
-
Endothelial FoxM1 reactivates aging-impaired endothelial regeneration for vascular repair and resolution of inflammatory lung injury.Sci Transl Med. 2023 Aug 16;15(709):eabm5755. doi: 10.1126/scitranslmed.abm5755. Epub 2023 Aug 16. Sci Transl Med. 2023. PMID: 37585502 Free PMC article.
-
Transgenic expression of FoxM1 promotes endothelial repair following lung injury induced by polymicrobial sepsis in mice.PLoS One. 2012;7(11):e50094. doi: 10.1371/journal.pone.0050094. Epub 2012 Nov 20. PLoS One. 2012. PMID: 23185540 Free PMC article.
-
Multiplicity of hypoxia-inducible transcription factors and their connection to the circadian clock in the zebrafish.Physiol Biochem Zool. 2015 Mar-Apr;88(2):146-57. doi: 10.1086/679751. Epub 2015 Jan 14. Physiol Biochem Zool. 2015. PMID: 25730270 Review.
-
Hypoxia-Inducible Factor Signaling in Inflammatory Lung Injury and Repair.Cells. 2022 Jan 6;11(2):183. doi: 10.3390/cells11020183. Cells. 2022. PMID: 35053299 Free PMC article. Review.
Cited by
-
Pericyte-derived exosomal miR-210 improves mitochondrial function and inhibits lipid peroxidation in vascular endothelial cells after traumatic spinal cord injury by activating JAK1/STAT3 signaling pathway.J Nanobiotechnology. 2023 Nov 27;21(1):452. doi: 10.1186/s12951-023-02110-y. J Nanobiotechnology. 2023. PMID: 38012616 Free PMC article.
-
Mechanisms of Pulmonary Hypertension in Acute Respiratory Distress Syndrome (ARDS).Front Mol Biosci. 2021 Jan 18;7:624093. doi: 10.3389/fmolb.2020.624093. eCollection 2020. Front Mol Biosci. 2021. PMID: 33537342 Free PMC article. Review.
-
Influencing Endothelial Cells' Roles in Inflammation and Wound Healing Through Nucleic Acid Delivery.Tissue Eng Part A. 2024 Apr;30(7-8):272-286. doi: 10.1089/ten.TEA.2023.0296. Epub 2024 Feb 7. Tissue Eng Part A. 2024. PMID: 38149606 Free PMC article. Review.
-
Expression and Regulation of Hypoxia-Inducible Factor Signalling in Acute Lung Inflammation.Cells. 2024 Dec 30;14(1):29. doi: 10.3390/cells14010029. Cells. 2024. PMID: 39791730 Free PMC article. Review.
-
The Interplay between Aquaporin-1 and the Hypoxia-Inducible Factor 1α in a Lipopolysaccharide-Induced Lung Injury Model in Human Pulmonary Microvascular Endothelial Cells.Int J Mol Sci. 2022 Sep 13;23(18):10588. doi: 10.3390/ijms231810588. Int J Mol Sci. 2022. PMID: 36142499 Free PMC article.
References
-
- Cines D.B., Pollak E.S., Buck C.A., Loscalzo J., Zimmerman G.A., McEver R.P., Pober J.S., Wick T.M., Konkle B.A., Schwartz B.S., Barnathan E.S., McCrae K.R., Hug B.A., Schmidt A.M., Stern D.M. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. - PubMed
-
- Deanfield J.E., Halcox J.P., Rabelink T.J. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. - PubMed
-
- Aird W.C. Phenotypic heterogeneity of the endothelium, I: structure, function, and mechanisms. Circ Res. 2007;100:158–173. - PubMed
-
- Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. - PubMed
-
- Austin G.E., Ratliff N.B., Hollman J., Tabei S., Phillips D.F. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985;6:369–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

