Exploitation of glycosylation in enveloped virus pathobiology
- PMID: 31121217
- PMCID: PMC6686077
- DOI: 10.1016/j.bbagen.2019.05.012
Exploitation of glycosylation in enveloped virus pathobiology
Abstract
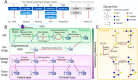
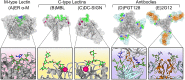
Glycosylation is a ubiquitous post-translational modification responsible for a multitude of crucial biological roles. As obligate parasites, viruses exploit host-cell machinery to glycosylate their own proteins during replication. Viral envelope proteins from a variety of human pathogens including HIV-1, influenza virus, Lassa virus, SARS, Zika virus, dengue virus, and Ebola virus have evolved to be extensively glycosylated. These host-cell derived glycans facilitate diverse structural and functional roles during the viral life-cycle, ranging from immune evasion by glycan shielding to enhancement of immune cell infection. In this review, we highlight the imperative and auxiliary roles glycans play, and how specific oligosaccharide structures facilitate these functions during viral pathogenesis. We discuss the growing efforts to exploit viral glycobiology in the development of anti-viral vaccines and therapies.
Keywords: Glycan shielding; Glycoprotein; Glycosylation; Structure; Virus; Virus-host interactions.
Copyright © 2019 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Cao L., Pauthner M., Andrabi R., Rantalainen K., Berndsen Z., Diedrich J.K., Menis S., Sok D., Bastidas R., Park S.-K.R., Delahunty C.M., He L., Guenaga J., Wyatt R.T., Schief W.R., Ward A.B., Yates J.R., Burton D.R., Paulson J.C. Differential processing of HIV envelope glycans on the virus and soluble recombinant trimer. Nat. Commun. 2018;9:3693. doi: 10.1038/s41467-018-06121-4. - DOI - PMC - PubMed
-
- Struwe W.B., Chertova E., Allen J.D., Seabright G.E., Watanabe Y., Harvey D.J., Medina-Ramirez M., Roser J.D., Smith R., Westcott D., Keele B.F., Bess J.W., Sanders R.W., Lifson J.D., Moore J.P., Crispin M. Site-specific glycosylation of virion-derived HIV-1 Env Is mimicked by a soluble trimeric immunogen. Cell Rep. 2018;24:1958–1966.e5. doi: 10.1016/j.celrep.2018.07.080. - DOI - PMC - PubMed
-
- Panico M., Bouché L., Binet D., O'Connor M.-J., Rahman D., Pang P.-C., Canis K., North S.J., Desrosiers R.C., Chertova E., Keele B.F., Bess J.W., Lifson J.D., Haslam S.M., Dell A., Morris H.R. Mapping the complete glycoproteome of virion-derived HIV-1 gp120 provides insights into broadly neutralizing antibody binding. Sci. Rep. 2016;6:32956. doi: 10.1038/srep32956. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

