Functional Connectivity of the Parasubiculum and Its Role in Temporal Lobe Epilepsy
- PMID: 31121261
- PMCID: PMC6597295
- DOI: 10.1016/j.neuroscience.2019.05.008
Functional Connectivity of the Parasubiculum and Its Role in Temporal Lobe Epilepsy
Abstract
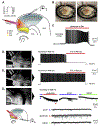
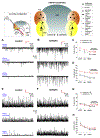
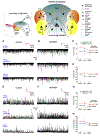
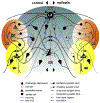
Temporal lobe epilepsy (TLE) is the commonest of adult epilepsies, often refractory to antiepileptic medications, whose prevention and treatment rely on understanding basic pathophysiological mechanisms in interlinked structures of the temporal lobe. The medial entorhinal area (MEA) is affected in TLE but mechanisms underlying hyperexcitability of MEA neurons require further elucidation. Previous studies have examined the role of the presubiculum (PrS) in mediating MEA pathophysiology but not the juxtaposed parasubiculum (Par). Here, we report on an electrophysiological assessment of the cells and circuits of the Par, their excitability under normal and epileptic conditions, and alterations in functional connectivity with neighboring PrS and MEA using the rat pilocarpine model of TLE. We show that Par, unlike the cell heterogeneous PrS, has a single dominant neuronal population whose excitability under epileptic conditions is altered by changes in both intrinsic properties and synaptic drive. These neurons experience significant reductions in synaptic inhibition and perish under chronic epileptic conditions. Connectivity between brain regions was deduced through changes in excitatory and inhibitory synaptic drive to neurons recorded in one region upon focal application of glutamate followed by NBQX to neurons in another using a microfluidic technique called CESOP and TLE-related circuit reorganization was assessed using data from normal and epileptic animals. The region-specific changes in Par and neighboring PrS and MEA together with their unexpected interactions are of significance in identifying ictogenic cells and circuits within the parahippocampal region and in unraveling pathophysiological mechanisms underlying TLE.
Keywords: CESOP; MEA; TLE; hyperexcitability; parasubiculum; presubiculum.
Copyright © 2019 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures






References
-
- Abbasi S, Kumar SS (2013), Electrophysiological and morphological characterization of cells in superficial layers of rat presubiculum. J Comp Neurol 521:3116–3132. - PubMed
-
- Alonso A, Llinas RR (1989), Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature 342:175–177. - PubMed
-
- Bear J, Fountain NB, Lothman EW (1996), Responses of the superficial entorhinal cortex in vitro in slices from naive and chronically epileptic rats. J Neurophysiol 76:2928–2940. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

