The NLRP1 inflammasome: new mechanistic insights and unresolved mysteries
- PMID: 31121538
- PMCID: PMC6800612
- DOI: 10.1016/j.coi.2019.04.015
The NLRP1 inflammasome: new mechanistic insights and unresolved mysteries
Abstract
Nucleotide-binding domain, leucine-rich repeat (NLR) proteins constitute a diverse class of innate immune sensors that detect pathogens or stress-associated stimuli in plants and animals. Some NLRs are activated upon direct binding to pathogen-derived ligands. In contrast, we focus here on a vertebrate NLR called NLRP1 that responds to the enzymatic activities of pathogen effectors. We discuss a newly proposed 'functional degradation' mechanism that explains activation and assembly of NLRP1 into an oligomeric complex called an inflammasome. We also discuss how NLRP1 is activated by non-pathogen-associated triggers such as the anti-cancer drug Val-boroPro, or by human disease-associated mutations. Finally, we discuss how research on NLRP1 has led to additional biological insights, including the unexpected discovery of a new CARD8 inflammasome.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures

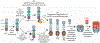


References
-
- Janeway CA Jr.: Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 1989, 54 Pt 1:1–13. - PubMed
-
- Jones JD, Dangl JL: The plant immune system. Nature 2006, 444:323–329. - PubMed
-
- Jones JD, Vance RE, Dangl JL: Intracellular innate immune surveillance devices in plants and animals. Science 2016, 354. - PubMed
-
- Martinon F, Burns K, Tschopp J: The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002, 10:417–426. - PubMed

