Quantifying Cancer Epithelial-Mesenchymal Plasticity and its Association with Stemness and Immune Response
- PMID: 31121840
- PMCID: PMC6572429
- DOI: 10.3390/jcm8050725
Quantifying Cancer Epithelial-Mesenchymal Plasticity and its Association with Stemness and Immune Response
Abstract
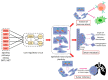
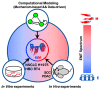
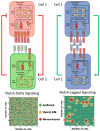
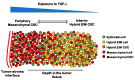
Cancer cells can acquire a spectrum of stable hybrid epithelial/mesenchymal (E/M) states during epithelial-mesenchymal transition (EMT). Cells in these hybrid E/M phenotypes often combine epithelial and mesenchymal features and tend to migrate collectively commonly as small clusters. Such collectively migrating cancer cells play a pivotal role in seeding metastases and their presence in cancer patients indicates an adverse prognostic factor. Moreover, cancer cells in hybrid E/M phenotypes tend to be more associated with stemness which endows them with tumor-initiation ability and therapy resistance. Most recently, cells undergoing EMT have been shown to promote immune suppression for better survival. A systematic understanding of the emergence of hybrid E/M phenotypes and the connection of EMT with stemness and immune suppression would contribute to more effective therapeutic strategies. In this review, we first discuss recent efforts combining theoretical and experimental approaches to elucidate mechanisms underlying EMT multi-stability (i.e., the existence of multiple stable phenotypes during EMT) and the properties of hybrid E/M phenotypes. Following we discuss non-cell-autonomous regulation of EMT by cell cooperation and extracellular matrix. Afterwards, we discuss various metrics that can be used to quantify EMT spectrum. We further describe possible mechanisms underlying the formation of clusters of circulating tumor cells. Last but not least, we summarize recent systems biology analysis of the role of EMT in the acquisition of stemness and immune suppression.
Keywords: CTC clusters; EMT metrics; EMT spectrum; epithelial–mesenchymal transition; hybrid epithelial/mesenchymal phenotypes; immune suppression; stemness; systems biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

