Nuclear Phospho-SOD1 Protects DNA from Oxidative Stress Damage in Amyotrophic Lateral Sclerosis
- PMID: 31121901
- PMCID: PMC6572067
- DOI: 10.3390/jcm8050729
Nuclear Phospho-SOD1 Protects DNA from Oxidative Stress Damage in Amyotrophic Lateral Sclerosis
Abstract
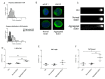
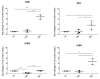
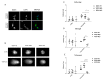
We already demonstrated that in peripheral blood mononuclear cells (PBMCs) of sporadic amyotrophic lateral sclerosis (sALS) patients, superoxide dismutase 1 (SOD1) was present in an aggregated form in the cytoplasmic compartment. Here, we investigated the possible effect of soluble SOD1 decrease and its consequent aggregation. We found an increase in DNA damage in patients PBMCs characterized by a high level of aggregated SOD1, while we found no DNA damage in PBMCs with normal soluble SOD1. We found an activation of ataxia-telangiectasia-mutated (ATM)/Chk2 and ATM and Rad3-related (ATR)/Chk1 DNA damage response pathways, which lead to phosphorylation of SOD1. Moreover, data showed that phosphorylation allows SOD1 to shift from the cytoplasm to the nucleus, protecting DNA from oxidative damage. Such pathway was finally confirmed in our cellular model. Our data lead us to suppose that in a sub-group of patients this physiologic pathway is non-functional, leading to an accumulation of DNA damage that causes the death of particularly susceptible cells, like motor neurons. In conclusion, during oxidative stress SOD1 is phosphorylated by Chk2 leading to its translocation in the nuclear compartment, in which SOD1 protects DNA from oxidative damage. This pathway, inefficient in sALS patients, could represent an innovative therapeutic target.
Keywords: ALS; DNA damage; SOD1; oxidative stress; peripheral blood mononuclear cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

