Application of Capillary Electrophoresis with Laser-Induced Fluorescence to Immunoassays and Enzyme Assays
- PMID: 31121978
- PMCID: PMC6571882
- DOI: 10.3390/molecules24101977
Application of Capillary Electrophoresis with Laser-Induced Fluorescence to Immunoassays and Enzyme Assays
Abstract
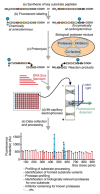
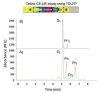
Capillary electrophoresis using laser-induced fluorescence detection (CE-LIF) is one of the most sensitive separation tools among electrical separation methods. The use of CE-LIF in immunoassays and enzyme assays has gained a reputation in recent years for its high detection sensitivity, short analysis time, and accurate quantification. Immunoassays are bioassay platforms that rely on binding reactions between an antigen (analyte) and a specific antibody. Enzyme assays measure enzymatic activity through quantitative analysis of substrates and products by the reaction of enzymes in purified enzyme or cell systems. These two category analyses play an important role in the context of biopharmaceutical analysis, clinical therapy, drug discovery, and diagnosis analysis. This review discusses the expanding portfolio of immune and enzyme assays using CE-LIF and focuses on the advantages and disadvantages of these methods over the ten years of existing technology since 2008.
Keywords: CE-LIF; chip-based CE-LIF assay; enzyme assay; immunoassay.
Conflict of interest statement
The authors declare no conflict of interest
Figures
Similar articles
-
[Advances in on-line enzyme assays by sequence analysis-based capillary electrophoresis].Se Pu. 2020 Oct 8;38(10):1143-1153. doi: 10.3724/SP.J.1123.2020.05008. Se Pu. 2020. PMID: 34213111 Review. Chinese.
-
Capillary electrophoresis coupled with laser-induced fluorescence polarization as a hybrid approach to ultrasensitive immunoassays.J Chromatogr A. 1999 Aug 20;853(1-2):555-62. doi: 10.1016/s0021-9673(99)00711-6. J Chromatogr A. 1999. PMID: 10486766
-
Human neutrophil elastase inhibition studied by capillary electrophoresis with laser induced fluorescence detection and microscale thermophoresis.J Chromatogr A. 2016 Jan 29;1431:215-223. doi: 10.1016/j.chroma.2015.12.079. Epub 2016 Jan 3. J Chromatogr A. 2016. PMID: 26777089
-
Capillary electrophoretic competitive immunoassay with laser-induced fluorescence detection for methionine-enkephalin.J Chromatogr A. 2006 Apr 14;1111(2):133-8. doi: 10.1016/j.chroma.2005.06.031. Epub 2005 Jul 5. J Chromatogr A. 2006. PMID: 16569571
-
Laser-Induced Fluorometry for Capillary Electrophoresis.Chem Rec. 2019 Feb;19(2-3):452-461. doi: 10.1002/tcr.201800051. Epub 2018 Aug 6. Chem Rec. 2019. PMID: 30079538 Review.
Cited by
-
Simple Sequence Repeat-Based Genetic Diversity Analysis of Alfalfa Varieties.Int J Mol Sci. 2025 May 29;26(11):5246. doi: 10.3390/ijms26115246. Int J Mol Sci. 2025. PMID: 40508055 Free PMC article.
-
Comparison of efficacy and safety of different asparaginases in adult acute lymphoblastic leukemia based on nano-magnetic beads immunoassay.Am J Transl Res. 2024 Jul 15;16(7):2931-2939. doi: 10.62347/CQGK2579. eCollection 2024. Am J Transl Res. 2024. PMID: 39114732 Free PMC article.
-
Moving Beyond DNA Sequence to Improve Plant Stress Responses.Front Genet. 2022 Apr 19;13:874648. doi: 10.3389/fgene.2022.874648. eCollection 2022. Front Genet. 2022. PMID: 35518351 Free PMC article. Review.
-
How can I measure brain acetylcholine levels in vivo? Advantages and caveats of commonly used approaches.J Neurochem. 2023 Oct;167(1):3-15. doi: 10.1111/jnc.15943. Epub 2023 Aug 24. J Neurochem. 2023. PMID: 37621094 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

