Effect of omega-3 plus methylphenidate as an alternative therapy to reduce attention deficit-hyperactivity disorder in children
- PMID: 31122010
- PMCID: PMC6753311
- DOI: 10.3345/kjp.2018.06982
Effect of omega-3 plus methylphenidate as an alternative therapy to reduce attention deficit-hyperactivity disorder in children
Abstract
Background: Attention deficit-hyperactivity disorder (ADHD) is one of the most common chronic behavioral disorders in school-aged children.
Purpose: This study aimed to evaluate the effect of omega-3 supplementation as an alternative therapy for ADHD, which can be caused by vitamin and mineral deficiencies.
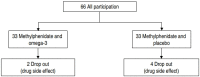
Methods: This was a double-blinded clinical trial study. Sixty-six children with ADHD (aged 6-12 years) referred to our child and adolescent psychiatric educational and therapeutic clinic were selected based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria. Instruments including the Parent ADHD Rating Scale were used to assess ADHD at 0, 2, 4, and 8 weeks during the study.
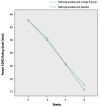
Results: The results showed no statistically significant difference between the methylphenidate with omega-3 group and methylphenidate with placebo group based on the Parents ADHD Rating Scale between week 0 (P≥0.96) and week 8 (P≥0.75). There were no significant intergroup differences between the Inattention (P≥0.48) and hyperactivity/impulsivity (P≥0.80) subscale scores on the Parents ADHD Rating Scale. The most common drug complications in the methylphenidate with placebo and methylphenidate with omega-3 groups were anorexia (27 [54%] vs. 41 [60.29%], respectively) and diarrhea (10 [20%] vs. 8 [11.76%], respectively), but the differences were not statistically significant (P> 0.05).
Conclusion: Our results demonstrate that a specific dose of omega-3 for 8 weeks had no effect on ADHD.
Keywords: Attention deficit-hyperactivity disorder; Child; Omega-3 supplement.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Randomized, Double-Blind, Placebo-Controlled Acute Comparator Trials of Lisdexamfetamine and Extended-Release Methylphenidate in Adolescents With Attention-Deficit/Hyperactivity Disorder.CNS Drugs. 2017 Nov;31(11):999-1014. doi: 10.1007/s40263-017-0468-2. CNS Drugs. 2017. PMID: 28980198 Free PMC article.
-
An Open-Label, Randomized Trial of Methylphenidate and Atomoxetine Treatment in Children with Attention-Deficit/Hyperactivity Disorder.J Child Adolesc Psychopharmacol. 2015 Sep;25(7):566-73. doi: 10.1089/cap.2015.0035. Epub 2015 Jul 29. J Child Adolesc Psychopharmacol. 2015. PMID: 26222447 Clinical Trial.
-
Does omega-3 supplement enhance the therapeutic results of methylphenidate in attention deficit hyperactivity disorder patients?J Res Med Sci. 2013 Aug;18(8):653-8. J Res Med Sci. 2013. PMID: 24379840 Free PMC article.
-
The efficacy and safety profile of lisdexamfetamine dimesylate, a prodrug of d-amphetamine, for the treatment of attention-deficit/hyperactivity disorder in children and adults.Clin Ther. 2009 Jan;31(1):142-76. doi: 10.1016/j.clinthera.2009.01.015. Clin Ther. 2009. PMID: 19243715 Review.
-
Do Omega-3/6 Fatty Acids Have a Therapeutic Role in Children and Young People with ADHD?J Lipids. 2017;2017:6285218. doi: 10.1155/2017/6285218. Epub 2017 Aug 30. J Lipids. 2017. PMID: 28951787 Free PMC article. Review.
Cited by
-
The stress-Wnt-signaling axis: a hypothesis for attention-deficit hyperactivity disorder and therapy approaches.Transl Psychiatry. 2020 Sep 18;10(1):315. doi: 10.1038/s41398-020-00999-9. Transl Psychiatry. 2020. PMID: 32948744 Free PMC article. Review.
-
Use of Non-Pharmacological Supplementations in Children and Adolescents with Attention Deficit/Hyperactivity Disorder: A Critical Review.Nutrients. 2020 May 28;12(6):1573. doi: 10.3390/nu12061573. Nutrients. 2020. PMID: 32481502 Free PMC article. Review.
-
Safety and efficacy of antioxidant therapy in children and adolescents with attention deficit hyperactivity disorder: A systematic review and network meta-analysis.PLoS One. 2024 Mar 28;19(3):e0296926. doi: 10.1371/journal.pone.0296926. eCollection 2024. PLoS One. 2024. PMID: 38547138 Free PMC article.
-
Efficacy and Safety of Polyunsaturated Fatty Acids Supplementation in the Treatment of Attention Deficit Hyperactivity Disorder (ADHD) in Children and Adolescents: A Systematic Review and Meta-Analysis of Clinical Trials.Nutrients. 2021 Apr 8;13(4):1226. doi: 10.3390/nu13041226. Nutrients. 2021. PMID: 33917727 Free PMC article.
-
Antioxidants as a Potential Target against Inflammation and Oxidative Stress in Attention-Deficit/Hyperactivity Disorder.Antioxidants (Basel). 2020 Feb 21;9(2):176. doi: 10.3390/antiox9020176. Antioxidants (Basel). 2020. PMID: 32098021 Free PMC article. Review.
References
-
- Antai-Otong D, Zimmerman ML. Treatment approaches to attention deficit hyperactivity disorder. Nurs Clin North Am. 2016;51:199–211. - PubMed
-
- Connor DF. Preschool attention deficit hyperactivity disorder: a review of prevalence, diagnosis, neurobiology, and stimulant treatment. J Dev Behav Pediatr. 2002;23(1 uppl):S1–9. - PubMed
-
- Rowland AS, Lesesne CA, Abramowitz AJ. The epidemiology of attention-deficit/hyperactivity disorder (ADHD): a public health view. Ment Retard Dev Disabil Res Rev. 2002;8:162–70. - PubMed
-
- Mick E, Biederman J, Prince J, Fischer MJ, Faraone SV. Impact of low birth weight on attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2002;23:16–22. - PubMed
-
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolesc Psychiatry. 2002;41:378–85. - PubMed
LinkOut - more resources
Full Text Sources