The LACOG-0415 phase II trial: abiraterone acetate and ADT versus apalutamide versus abiraterone acetate and apalutamide in patients with advanced prostate cancer with non-castration testosterone levels
- PMID: 31122212
- PMCID: PMC6533731
- DOI: 10.1186/s12885-019-5709-y
The LACOG-0415 phase II trial: abiraterone acetate and ADT versus apalutamide versus abiraterone acetate and apalutamide in patients with advanced prostate cancer with non-castration testosterone levels
Abstract
Background: Testosterone suppression is the standard treatment for advanced prostate cancer, and it is associated with side-effects that impair patients' quality of life, like sexual dysfunction, osteoporosis, weight gain, and increased cardiovascular risk. We hypothesized that abiraterone acetate with prednisone (AAP) and apalutamide, alone or in combination, can be an effective hormonal therapy also possibly decreasing castration-associated side effects.
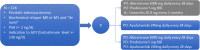
Methods: Phase II, open-label, randomized, efficacy trial of abiraterone acetate plus prednisone (AAP) and Androgen Deprivation Therapy (ADT) versus apalutamide versus the combination of AAP (without ADT) and apalutamide. Key eligibility criteria are confirmed prostate adenocarcinoma; biochemical relapse after definitive treatment (PSA ≥ 4 ng/ml and doubling time less than 10 months, or PSA ≥ 20 ng/ml); newly diagnosed locally advanced or metastatic prostate cancer; asymptomatic to moderately symptomatic regarding bone symptoms. Patients with other histology besides adenocarcinoma or previous use of hormonal therapy or chemotherapy were excluded.
Discussion: There is an urgent need to study and validate regimens such as new hormonal agents that may add benefit to castration with an acceptable safety profile. We aim to evaluate if apalutamide in monotherapy or in combination with AAP is an effective and safety hormonal treatment that can spare patients of androgen deprivation therapy.
Trial registration: This trial was registered in ClinicalTrials.gov on October 16, 2017, under Identifier: NCT02867020.
Keywords: Abiraterone; Androgen deprivation therapy; Apalutamide; Castration-sensitive prostate cancer; Goserelin; Hormonal therapy.
Conflict of interest statement
This study has received funding from a commercial organization. Otherwise, the authors declare that they have no competing interests.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous