Thermal manipulation of the broilers embryos: expression of muscle markers genes and weights of body and internal organs during embryonic and post-hatch days
- PMID: 31122240
- PMCID: PMC6533759
- DOI: 10.1186/s12917-019-1917-6
Thermal manipulation of the broilers embryos: expression of muscle markers genes and weights of body and internal organs during embryonic and post-hatch days
Abstract
Background: In broilers chickens, the molecular bases for promoting muscle development and growth requires further investigation. Therefore, the current study aimed to investigate the effects of daily thermal manipulation (TM) during embryonic days (ED) 12 to 18 on body, carcass and internal organ weights as well as on the expression of muscle growth markers genes during late embryogenesis and post-hatch days. 1500 fertile Cobb eggs were divided into five groups. The first group was a control group and incubated at 37.8°C. The other four groups were thermally manipulated (TM) and exposed to 38.5°C (TM1), 39°C (TM2), 39.5°C (TM3) and 40°C (TM4) daily for 18 h, respectively, with a relative humidity of 56%. Body weights (BW) from ED 12 to 18 and on post-hatch days 1, 2, 3, 4, 5, 6, 7, 14, 21, 28 and 35 were recorded. mRNA expression levels of muscle growth factor genes (IGF-1 and GH) and muscle marker genes (Myogenic Differentiation Antigen; MyoD), Myogenin, Pax7, and PCNA) during ED 12 to 18 and on post-hatch days 1, 3, 5, 7, 14 were analyzed. On post-hatch day 35, the carcass and internal organ weights have been also evaluated.
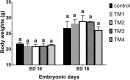
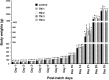
Results: TM during certain days of embryogenesis (ED 12 to 18) did not affect the BW of broilers during their embryonic lives. However, TM, particularly TM1 and TM2, significantly increased BW, carcass and internal weights of hatched chicks near to the marketing age (post-hatch days 28 and 35). Most of TM protocols induced up-regulation of muscle growth factor genes (IGF-1 and GH) and muscle marker genes (MyoD, Myogenin, Pax7, and PCNA) during embryonic life (ED 12 to 18) and on post-hatch days.
Conclusion: Among the various TM conditions, it seems that,TM1 and TM2 induced a significant increase in BW, carcass and internal weights of hatched chicks near to the marketing age. This increase in BW induced presumably via up-regulation of muscle growth factor genes and muscle growth markers genes during embryonic life (ED 12 to 18) and on post-hatch days. Both protocols (TM1 and TM2) can be used in real-world applications of poultry industry for maximum benefit.
Keywords: Broiler; Growth factor; Marker gene; Muscle; Thermal manipulation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Basal and dynamics mRNA expression of muscular HSP108, HSP90, HSF-1 and HSF-2 in thermally manipulated broilers during embryogenesis.BMC Vet Res. 2019 Mar 8;15(1):83. doi: 10.1186/s12917-019-1827-7. BMC Vet Res. 2019. PMID: 30849975 Free PMC article.
-
Biochemical and molecular investigation of thermal manipulation protocols during broiler embryogenesis and subsequent thermal challenge.BMC Vet Res. 2015 Dec 1;11:292. doi: 10.1186/s12917-015-0609-0. BMC Vet Res. 2015. PMID: 26627061 Free PMC article.
-
Thermal manipulation during chicken embryogenesis results in enhanced Hsp70 gene expression and the acquisition of thermotolerance.Res Vet Sci. 2013 Oct;95(2):502-7. doi: 10.1016/j.rvsc.2013.05.012. Epub 2013 Jun 18. Res Vet Sci. 2013. PMID: 23787299 Clinical Trial.
-
Embryonic thermal manipulation of poultry birds: Lucrative and deleterious effects.J Anim Physiol Anim Nutr (Berl). 2024 Mar;108(2):346-356. doi: 10.1111/jpn.13896. Epub 2023 Oct 26. J Anim Physiol Anim Nutr (Berl). 2024. PMID: 37885333 Review.
-
Myogenesis--possibilities of its stimulation in chickens.Folia Biol (Krakow). 2011;59(3-4):85-90. doi: 10.3409/fb59_3-4.85-90. Folia Biol (Krakow). 2011. PMID: 22195459 Review.
Cited by
-
Thermal manipulation and rosemary extract: Their combined effects on embryonic development, hatchability, and physiological responses of hatched chicks.Vet Anim Sci. 2025 Feb 28;27:100435. doi: 10.1016/j.vas.2025.100435. eCollection 2025 Mar. Vet Anim Sci. 2025. PMID: 40124401 Free PMC article.
-
Thermal Manipulation During the Embryonic Stage and the Post-Hatch Characteristics of Broiler Chickens.Animals (Basel). 2024 Nov 27;14(23):3436. doi: 10.3390/ani14233436. Animals (Basel). 2024. PMID: 39682400 Free PMC article.
-
Embryonic Thermal Manipulation Affects the Antioxidant Response to Post-Hatch Thermal Exposure in Broiler Chickens.Animals (Basel). 2020 Jan 13;10(1):126. doi: 10.3390/ani10010126. Animals (Basel). 2020. PMID: 31941014 Free PMC article.
-
Embryonic modulation through thermal manipulation and in ovo feeding to develop heat tolerance in chickens.Anim Nutr. 2023 Jan 17;13:150-159. doi: 10.1016/j.aninu.2023.01.005. eCollection 2023 Jun. Anim Nutr. 2023. PMID: 37123616 Free PMC article. Review.
-
The Role of Incubation Conditions on the Regulation of Muscle Development and Meat Quality in Poultry.Front Physiol. 2022 Jun 15;13:883134. doi: 10.3389/fphys.2022.883134. eCollection 2022. Front Physiol. 2022. PMID: 35784883 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

