Microtubule Assembly from Single Flared Protofilaments-Forget the Cozy Corner?
- PMID: 31122668
- PMCID: PMC6588822
- DOI: 10.1016/j.bpj.2019.05.005
Microtubule Assembly from Single Flared Protofilaments-Forget the Cozy Corner?
Abstract
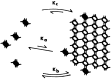
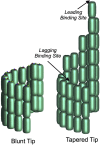
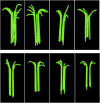
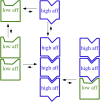
A paradigm shift for models of MT assembly is suggested by a recent cryo-electron microscopy study of microtubules (MTs). Previous assembly models have been based on the two-dimensional lattice of the MT wall, where incoming subunits can add with longitudinal and lateral bonds. The new study of McIntosh et al. concludes that the growing ends of MTs separate into flared single protofilaments. This means that incoming subunits must add onto the end of single protofilaments, forming only a longitudinal bond. How can growth of single-stranded protofilaments exhibit cooperative assembly with a critical concentration? An answer is suggested by FtsZ, the bacterial tubulin homolog, which assembles into single-stranded protofilaments. Cooperative assembly of FtsZ is thought to be based on conformational changes that switch the longitudinal bond from low to high affinity when the subunit is incorporated in a protofilament. This novel mechanism may also apply to tubulin assembly and could be the primary mechanism for assembly onto single flared protofilaments.
Copyright © 2019 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- McIntosh J.R., O’Toole E., Gudimchuk N. Microtubules grow by the addition of bent guanosine triphosphate tubulin to the tips of curved protofilaments. J. Cell Biol. 2018;217:2691–2708. - PMC - PubMed
- McIntosh, J. R., E. O’Toole, …, N. Gudimchuk. 2018. Microtubules grow by the addition of bent guanosine triphosphate tubulin to the tips of curved protofilaments. J. Cell Biol. 217:2691-2708. - PMC - PubMed
-
- Erickson H.P., Pantaloni D. The role of subunit entropy in cooperative assembly. Nucleation of microtubules and other two-dimensional polymers. Biophys. J. 1981;34:293–309. - PMC - PubMed
- Erickson, H. P., and D. Pantaloni. 1981. The role of subunit entropy in cooperative assembly. Nucleation of microtubules and other two-dimensional polymers. Biophys. J. 34:293-309. - PMC - PubMed
-
- Erickson H.P. Co-operativity in protein-protein association. The structure and stability of the actin filament. J. Mol. Biol. 1989;206:465–474. - PubMed
- Erickson, H. P. 1989. Co-operativity in protein-protein association. The structure and stability of the actin filament. J. Mol. Biol. 206:465-474. - PubMed
-
- Chrétien D., Fuller S.D., Karsenti E. Structure of growing microtubule ends: two-dimensional sheets close into tubes at variable rates. J. Cell Biol. 1995;129:1311–1328. - PMC - PubMed
- Chretien, D., S. D. Fuller, and E. Karsenti. 1995. Structure of growing microtubule ends: two-dimensional sheets close into tubes at variable rates. J. Cell Biol. 129:1311-1328. - PMC - PubMed
-
- VanBuren V., Odde D.J., Cassimeris L. Estimates of lateral and longitudinal bond energies within the microtubule lattice. Proc. Natl. Acad. Sci. USA. 2002;99:6035–6040. - PMC - PubMed
- VanBuren, V., D. J. Odde, and L. Cassimeris. 2002. Estimates of lateral and longitudinal bond energies within the microtubule lattice. Proc. Natl. Acad. Sci. USA. 99:6035-6040. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

