Neurodevelopmental effects of ante-partum and post-partum antiretroviral exposure in HIV-exposed and uninfected children versus HIV-unexposed and uninfected children in Uganda and Malawi: a prospective cohort study
- PMID: 31122797
- PMCID: PMC6677626
- DOI: 10.1016/S2352-3018(19)30083-9
Neurodevelopmental effects of ante-partum and post-partum antiretroviral exposure in HIV-exposed and uninfected children versus HIV-unexposed and uninfected children in Uganda and Malawi: a prospective cohort study
Abstract
Background: Antiretroviral medication during pregnancy and breastfeeding substantially decreases the risk of HIV transmission from mothers to infants, but its effects on the child's neurodevelopment are unknown. This study compared neurodevelopmental outcomes of ante-partum and post-partum antiretroviral exposure in HIV-exposed and uninfected children with HIV-unexposed and uninfected children at ages 12, 24, 48, and 60 months.
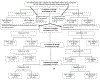
Methods: For this study, a prospective cohort of HIV-exposed and uninfected children was identified from two research sites in the PROMISE-BF trial (at Blantyre, Malawi, and Kampala, Uganda), in which pregnant HIV-infected mothers were randomly assigned to triple antiretroviral prophylaxis (lopinavir-ritonavir plus either lamivudine and zidovudine or emtricitabine and tenofovir), versus zidovudine alone. Post partum, the mother-infant pairs were randomly assigned to maternal triple antiretroviral treatment or infant nevirapine during breastfeeding. HIV-unexposed and uninfected children matched for age, sex, and socioeconomic background were enrolled at vaccination and well-child clinics at the study sites. We included only children without a history of documented brain infection or injury or substantial malnutrition, and whose mothers were randomly assigned and maintained within their assigned ante-partum and post-partum phases throughout their treatment arm periods. Primary outcomes were the Mullen Scales of Early Learning (MSEL) cognitive composite score at age 12 months, 24 months, and 48 months; and the mental processing index for the Kaufman Assessment Battery for Children, second edition (KABC-II) global score at 48 months and 60 months. Repeated measures were analysed using a linear mixed-effects model controlling for data collection site.
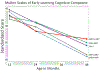
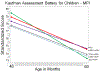
Findings: Between Aug 23, 2013, and Dec 17, 2014, we co-enrolled 861 children. For MSEL assessments, 738 were eligible for inclusion at age 12 months, 790 at age 24 months, and 692 at age 48 months. For KABC-II assessments, 685 were eligible for inclusion at age 48 months and 445 at age 60 months. There were no differences in MSEL cognitive composite scores according to exposure at age 12 and 24 months (p=0·19 and 0·24, respectively, for comparison of all groups). At 48 months, MSEL cognitive composite scores were worse for children of mothers who did not remain on triple antiretroviral treatment throughout both the ante-partum and post-partum treatment phases (adjusted means 80·64 [95% CI 77·74-83·54] and 81·34 [78·19-84·48], respectively), compared with those who did remain on triple treatment (adjusted mean 85·93, 95% CI 83·05-88·80; p=0·0486 for the comparison of all groups). The KABC-II composite scores (mental processing index) did not differ at 48 or 60 months according to exposure (p=0·81 and 0·89, respectively, for comparison of all groups). Scores for MSEL and KABC-II for children of mothers on triple antiretrovirals in both the ante-partum and post-partum treatment phases were similar to those for children in the HIV-unexposed and uninfected reference group at all timepoints.
Interpretation: Maternal triple antiretroviral exposure during both the ante-partum and post-partum phases did not result in greater developmental risks for the mothers' HIV-exposed and uninfected children through age 60 months, compared with children who were HIV-unexposed and uninfected. This might be because ante-partum triple antiretroviral protection of the health of mothers with HIV during pregnancy might be neuroprotective for the child, and when continued post partum, could enhance the quality of caregiving for the child through better clinical care for the mother.
Funding: National Institutes of Health and Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
All study authors declare no competing interests.
Figures
Comment in
-
Exposure of HIV-exposed uninfected infants to antiretrovirals.Lancet HIV. 2019 Aug;6(8):e487-e488. doi: 10.1016/S2352-3018(19)30161-4. Epub 2019 May 20. Lancet HIV. 2019. PMID: 31122796 No abstract available.
References
-
- Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr 2002; 29(5): 484–94. - PubMed
-
- Fowler MG, Mofenson LM, Taha TE. Antiretroviral Therapy for Perinatal HIV Prevention. N Engl J Med 2017; 376(7): 699–700. - PubMed
-
- Flynn PM, Taha TE, Cababasay M, et al. Prevention of HIV-1 Transmission Through Breastfeeding: Efficacy and Safety of Maternal Antiretroviral Therapy Versus Infant Nevirapine Prophylaxis for Duration of Breastfeeding in HIV-1-Infected Women With High CD4 Cell Count (IMPAACT PROMISE): A Randomized, Open-Label, Clinical Trial. J Acquir Immune Defic Syndr 2018; 77(4): 383–92. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

