PRC2 is high maintenance
- PMID: 31123062
- PMCID: PMC6672058
- DOI: 10.1101/gad.325050.119
PRC2 is high maintenance
Abstract
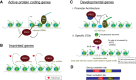
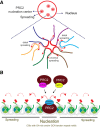
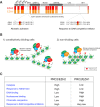
As the process that silences gene expression ensues during development, the stage is set for the activity of Polycomb-repressive complex 2 (PRC2) to maintain these repressed gene profiles. PRC2 catalyzes a specific histone posttranslational modification (hPTM) that fosters chromatin compaction. PRC2 also facilitates the inheritance of this hPTM through its self-contained "write and read" activities, key to preserving cellular identity during cell division. As these changes in gene expression occur without changes in DNA sequence and are inherited, the process is epigenetic in scope. Mutants of mammalian PRC2 or of its histone substrate contribute to the cancer process and other diseases, and research into these aberrant pathways is yielding viable candidates for therapeutic targeting. The effectiveness of PRC2 hinges on its being recruited to the proper chromatin sites; however, resolving the determinants to this process in the mammalian case was not straightforward and thus piqued the interest of many in the field. Here, we chronicle the latest advances toward exposing mammalian PRC2 and its high maintenance.
Keywords: PRC2; Polycomb; chromatin; epigenetics.
© 2019 Yu et al.; Published by Cold Spring Harbor Laboratory Press.
Figures





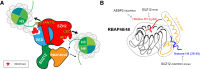




References
-
- Antonysamy S, Condon B, Druzina Z, Bonanno JB, Gheyi T, Zhang F, MacEwan I, Zhang A, Ashok S, Rodgers L, et al. 2013. Structural context of disease-associated mutations and putative mechanism of autoinhibition revealed by X-ray crystallographic analysis of the EZH2-SET domain. PLoS One 8: e84147 10.1371/journal.pone.0084147 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
