Epigenetics and transcription regulation during eukaryotic diversification: the saga of TFIID
- PMID: 31123066
- PMCID: PMC6672047
- DOI: 10.1101/gad.300475.117
Epigenetics and transcription regulation during eukaryotic diversification: the saga of TFIID
Abstract
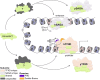
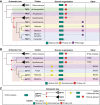
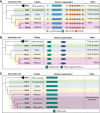
The basal transcription factor TFIID is central for RNA polymerase II-dependent transcription. Human TFIID is endowed with chromatin reader and DNA-binding domains and protein interaction surfaces. Fourteen TFIID TATA-binding protein (TBP)-associated factor (TAF) subunits assemble into the holocomplex, which shares subunits with the Spt-Ada-Gcn5-acetyltransferase (SAGA) coactivator. Here, we discuss the structural and functional evolution of TFIID and its divergence from SAGA. Our orthologous tree and domain analyses reveal dynamic gains and losses of epigenetic readers, plant-specific functions of TAF1 and TAF4, the HEAT2-like repeat in TAF2, and, importantly, the pre-LECA origin of TFIID and SAGA. TFIID evolution exemplifies the dynamic plasticity in transcription complexes in the eukaryotic lineage.
Keywords: SAGA; TFIID; basal transcription; phylogenetic analyses.
© 2019 Antonova et al.; Published by Cold Spring Harbor Laboratory Press.
Figures




Similar articles
-
TAF4 nucleates a core subcomplex of TFIID and mediates activated transcription from a TATA-less promoter.Proc Natl Acad Sci U S A. 2006 Aug 15;103(33):12347-52. doi: 10.1073/pnas.0605499103. Epub 2006 Aug 8. Proc Natl Acad Sci U S A. 2006. PMID: 16895980 Free PMC article.
-
SAGA and TFIID: Friends of TBP drifting apart.Biochim Biophys Acta Gene Regul Mech. 2021 Feb;1864(2):194604. doi: 10.1016/j.bbagrm.2020.194604. Epub 2020 Jul 14. Biochim Biophys Acta Gene Regul Mech. 2021. PMID: 32673655 Review.
-
Redundant roles for the TFIID and SAGA complexes in global transcription.Nature. 2000 Jun 8;405(6787):701-4. doi: 10.1038/35015104. Nature. 2000. PMID: 10864329
-
Genome-wide transcriptional dependence on TAF1 functional domains.J Biol Chem. 2006 Mar 10;281(10):6404-12. doi: 10.1074/jbc.M513776200. Epub 2006 Jan 2. J Biol Chem. 2006. PMID: 16407318
-
Distinct regulatory mechanisms of eukaryotic transcriptional activation by SAGA and TFIID.Biochim Biophys Acta. 2011 Feb;1809(2):97-108. doi: 10.1016/j.bbagrm.2010.08.009. Epub 2010 Aug 26. Biochim Biophys Acta. 2011. PMID: 20800707 Free PMC article. Review.
Cited by
-
The roles of TAF1 in neuroscience and beyond.R Soc Open Sci. 2024 Sep 25;11(9):240790. doi: 10.1098/rsos.240790. eCollection 2024 Sep. R Soc Open Sci. 2024. PMID: 39323550 Free PMC article. Review.
-
Structure and mechanism of the RNA polymerase II transcription machinery.Genes Dev. 2020 Apr 1;34(7-8):465-488. doi: 10.1101/gad.335679.119. Genes Dev. 2020. PMID: 32238450 Free PMC article. Review.
-
Dynamic and regulated TAF gene expression during mouse embryonic germ cell development.PLoS Genet. 2020 Jan 8;16(1):e1008515. doi: 10.1371/journal.pgen.1008515. eCollection 2020 Jan. PLoS Genet. 2020. PMID: 31914128 Free PMC article.
-
The Core Promoter Is a Regulatory Hub for Developmental Gene Expression.Front Cell Dev Biol. 2021 Sep 10;9:666508. doi: 10.3389/fcell.2021.666508. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34568311 Free PMC article. Review.
-
SUPT3H-less SAGA coactivator can assemble and function without significantly perturbing RNA polymerase II transcription in mammalian cells.Nucleic Acids Res. 2022 Aug 12;50(14):7972-7990. doi: 10.1093/nar/gkac637. Nucleic Acids Res. 2022. PMID: 35871303 Free PMC article.
References
-
- Bertrand C, Benhamed M, Li YF, Ayadi M, Lemonnier G, Renou JP, Delarue M, Zhou DX. 2005. Arabidopsis HAF2 gene encoding TATA-binding protein (TBP)-associated factor TAF1, is required to integrate light signals to regulate gene expression and growth. J Biol Chem 280: 1465–1473. 10.1074/jbc.M409000200 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
