Sugar-induced de novo cytokinin biosynthesis contributes to Arabidopsis growth under elevated CO2
- PMID: 31123308
- PMCID: PMC6533260
- DOI: 10.1038/s41598-019-44185-4
Sugar-induced de novo cytokinin biosynthesis contributes to Arabidopsis growth under elevated CO2
Abstract
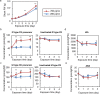
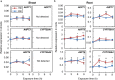
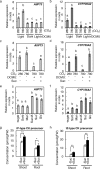
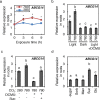
Carbon availability is a major regulatory factor in plant growth and development. Cytokinins, plant hormones that play important roles in various aspects of growth and development, have been implicated in the carbon-dependent regulation of plant growth; however, the details of their involvement remain to be elucidated. Here, we report that sugar-induced cytokinin biosynthesis plays a role in growth enhancement under elevated CO2 in Arabidopsis thaliana. Growing Arabidopsis seedlings under elevated CO2 resulted in an accumulation of cytokinin precursors that preceded growth enhancement. In roots, elevated CO2 induced two genes involved in de novo cytokinin biosynthesis: an adenosine phosphate-isopentenyltransferase gene, AtIPT3, and a cytochrome P450 monooxygenase gene, CYP735A2. The expression of these genes was inhibited by a photosynthesis inhibitor, DCMU, under elevated CO2, and was enhanced by sugar supplements, indicating that photosynthetically generated sugars are responsible for the induction. Consistently, cytokinin precursor accumulation was enhanced by sugar supplements. Cytokinin biosynthetic mutants were impaired in growth enhancement under elevated CO2, demonstrating the involvement of de novo cytokinin biosynthesis for a robust growth response. We propose that plants employ a system to regulate growth in response to elevated CO2 in which photosynthetically generated sugars induce de novo cytokinin biosynthesis for growth regulation.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

