A Chemical Biology View of Bioactive Small Molecules and a Binder-Based Approach to Connect Biology to Precision Medicines
- PMID: 31123369
- PMCID: PMC6528670
- DOI: 10.1002/ijch.201800113
A Chemical Biology View of Bioactive Small Molecules and a Binder-Based Approach to Connect Biology to Precision Medicines
Figures



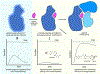
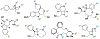
References
-
- Plenge RM, Scolnick EM, Altshuler D, Nat. Rev. Drug Discovery 2013, 12, 581–594. - PubMed
-
- Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA, The New England journal of medicine 2015, 372, 1500–1509. - PubMed
-
- Harding MW, Galat A, Uehling DE, Schreiber SL, Nature 1989, 341, 758–760. - PubMed
-
- Liu J, Farmer JD Jr., Lane WS, Friedman J, Weissman I, Schreiber SL, Cell 1991, 66, 807–815; - PubMed
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL, Nature 1994, 369, 756–758; - PubMed
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH, Cell 1994, 78, 35–43. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
