Separation of spermatozoa from erythrocytes using their tumbling mechanism in a pinch flow fractionation device
- PMID: 31123596
- PMCID: PMC6527678
- DOI: 10.1038/s41378-019-0068-z
Separation of spermatozoa from erythrocytes using their tumbling mechanism in a pinch flow fractionation device
Abstract
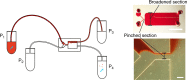
Men suffering from azoospermia can father a child, by extracting spermatozoa from a testicular biopsy sample. The main complication in this procedure is the presence of an abundance of erythrocytes. Currently, the isolation of the few spermatozoa from the sample is manually performed due to ineffectiveness of filtering methods, making it time consuming and labor intensive. The spermatozoa are smaller in both width and height than any other cell type found in the sample, with a very small difference compared with the erythrocyte for the smallest, making this not the feature to base the extraction on. However, the length of the spermatozoon is 5× larger than the diameter of an erythrocyte and can be utilized. Here we propose a microfluidic chip, in which the tumbling behavior of spermatozoa in pinched flow fractionation is utilized to separate them from the erythrocytes. We show that we can extract 95% of the spermatozoa from a sample containing 2.5% spermatozoa, while removing around 90% of the erythrocytes. By adjusting the flow rates, we are able to increase the collection efficiency while slightly sacrificing the purity, tuning the solution for the available sample in the clinic.
Keywords: Microfluidics; Nanobiotechnology.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
