Helicobacter pylori Deregulates T and B Cell Signaling to Trigger Immune Evasion
- PMID: 31123892
- PMCID: PMC7050993
- DOI: 10.1007/978-3-030-15138-6_10
Helicobacter pylori Deregulates T and B Cell Signaling to Trigger Immune Evasion
Abstract
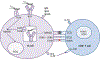
Helicobacter pylori is a prevalent human pathogen that successfully establishes chronic infection, which leads to clinically significant gastric diseases including chronic gastritis, peptic ulcer disease (PUD), and gastric cancer (GC). H. pylori is able to produce a persistent infection due in large part to its ability to hijack the host immune response. The host adaptive immune response is activated to strategically and specifically attack pathogens and normally clears them from the infected host. Since B and T lymphocytes are central mediators of adaptive immunity, in this chapter we review their development and the fundamental mechanisms regulating their activation in order to understand how some of the normal processes are subverted by H. pylori. In this review, we place particular emphasis on the CD4+ T cell responses, their subtypes, and regulatory mechanisms because of the expanding literature in this area related to H. pylori. T lymphocyte differentiation and function are finely orchestrated through a series of cell-cell interactions, which include immune checkpoint receptors. Among the immune checkpoint receptor family, there are some with inhibitory properties that are exploited by tumor cells to facilitate their immune evasion. Gastric epithelial cells (GECs), which act as antigen-presenting cells (APCs) in the gastric mucosa, are induced by H. pylori to express immune checkpoint receptors known to sway T lymphocyte function and thus circumvent effective T effector lymphocyte responses. This chapter reviews these and other mechanisms used by H. pylori to interfere with host immunity in order to persist.
Keywords: Co-inhibitory receptors; Immune checkpoint regulators; Immune evasion; Lymphocyte development; Reprogramming.
Figures




References
-
- Akhiani AA, Schon K, Franzen LE, Pappo J, Lycke N(2004) Helicobacter pylori-specific antibodies impair the development of gastritis, facilitate bacterial colonization, and counteract resistance against infection. J Immunol 172(8):5024–5033 - PubMed
-
- Akhiani AA, Stensson A, Schon K, Lycke NY (2005) IgA antibodies impair resistance against Helicobacter pylori infection: studies on immune evasion in IL-10-deficient mice. J Immunol 174(12):8144–8153 - PubMed
-
- Allen LA (1999) Intracellular niches for extracellular bacteria: lessons from Helicobacter pylori. J Leukoc Biol 66(5):753–756 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

