HIV-1 Tat promotes astrocytic release of CCL2 through MMP/PAR-1 signaling
- PMID: 31124192
- PMCID: PMC6640102
- DOI: 10.1002/glia.23642
HIV-1 Tat promotes astrocytic release of CCL2 through MMP/PAR-1 signaling
Abstract
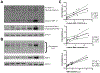
The HIV-1 protein Tat is continually released by HIV-infected cells despite effective combination antiretroviral therapies (cART). Tat promotes neurotoxicity through enhanced expression of proinflammatory molecules from resident and infiltrating immune cells. These molecules include matrix metalloproteinases (MMPs), which are pathologically elevated in HIV, and are known to drive central nervous system (CNS) injury in varied disease settings. A subset of MMPs can activate G-protein coupled protease-activated receptor 1 (PAR-1), a receptor that is highly expressed on astrocytes. Although PAR-1 expression is increased in HIV-associated neurocognitive disorder (HAND), its role in HAND pathogenesis remains understudied. Herein, we explored Tat's ability to induce expression of the PAR-1 agonists MMP-3 and MMP-13. We also investigated MMP/PAR-1-mediated release of CCL2, a chemokine that drives CNS entry of HIV infected monocytes and remains a significant correlate of cognitive dysfunction in the era of cART. Tat exposure significantly increased the expression of MMP-3 and MMP-13. These PAR-1 agonists both stimulated the release of astrocytic CCL2, and both genetic knock-out and pharmacological inhibition of PAR-1 reduced CCL2 release. Moreover, in HIV-infected post-mortem brain tissue, within-sample analyses revealed a correlation between levels of PAR-1-activating MMPs, PAR-1, and CCL2. Collectively, these findings identify MMP/PAR-1 signaling to be involved in the release of CCL2, which may underlie Tat-induced neuroinflammation.
Keywords: CCL2; HIV; Tat; matrix metalloproteinase; protease activated receptor 1.
© 2019 Wiley Periodicals, Inc.
Figures
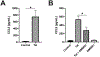
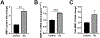



References
-
- Atluri VSR, Hidalgo M, Samikkannu T, Kurapati KRV, Jayant RD, Sagar V, Nair MPN. 2015. Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update. Front Cell Neurosci [Internet] 9 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4461820/ - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

