Effect of Fasudil on remyelination following cuprizone-induced demyelination
- PMID: 31124292
- PMCID: PMC6930827
- DOI: 10.1111/cns.13154
Effect of Fasudil on remyelination following cuprizone-induced demyelination
Retraction in
-
Retraction: Effect of Fasudil on remyelination following cuprizone-induced demyelination.CNS Neurosci Ther. 2020 Jul;26(7):778. doi: 10.1111/cns.13393. Epub 2020 May 27. CNS Neurosci Ther. 2020. PMID: 32462797 Free PMC article.
Abstract
Background: Multiple sclerosis is characterized by demyelination/remyelination, neuroinflammation, and neurodegeneration. Cuprizone (CPZ)-induced toxic demyelination is an experimental animal model commonly used to study demyelination and remyelination in the central nervous system. Fasudil is one of the most thoroughly studied Rho kinase inhibitors.
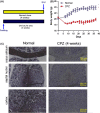
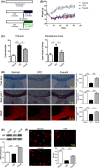
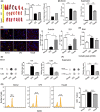
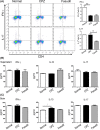
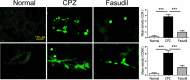
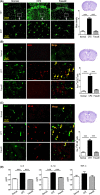
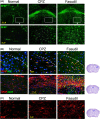
Methods: Following CPZ exposure, the degree of demyelination in the brain of male C57BL/6 mice was assessed by Luxol fast blue, Black Gold II, myelin basic protein immunofluorescent staining, and Western blot. The effect of Fasudil on behavioral change was determined using elevated plus maze test and pole test. The possible mechanisms of Fasudil action were examined by immunohistochemistry, flow cytometry, ELISA, and dot blot.
Results: Fasudil improved behavioral abnormalities, inhibited microglia-mediated neuroinflammation, and promoted astrocyte-derived nerve growth factor and ciliary neurotrophic factor, which should contribute to protection and regeneration of oligodendrocytes. In addition, Fasudil inhibited the production of myelin oligodendrocyte glycoprotein antibody and the infiltration of peripheral CD4+ T cells and CD68+ macrophages, which appears to be related to the integrity of the blood-brain barrier.
Conclusion: These results provide evidence for the therapeutic potential of Fasudil in CPZ-induced demyelination. However, how Fasudil acts on microglia, astrocytes, and immune cells remains to be further explored.
Keywords: Fasudil; Rho kinase; cuprizone-induced demyelination; remyelination.
© 2019 The Authors. CNS Neuroscience & Therapeutics Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures
References
-
- Bauer J, Rauschka H, Lassmann H. Inflammation in the nervous system: the human perspective. Glia. 2001;36(2):235‐243. - PubMed
-
- Benn T, Halfpenny C, Scolding N. Glial cells as targets for cytotoxic immune mediators. Glia. 2001;36(2):200‐211. - PubMed
-
- Mahurkar S, Suppiah V, O'Doherty C. Pharmacogenomics of interferon beta and glatiramer acetate response: a review of the literature. Autoimmun Rev. 2014;13(2):178‐186. - PubMed
-
- Koch JC, Tatenhorst L, Roser AE, Saal KA, Tonges L, Lingor P. ROCK inhibition in models of neurodegeneration and its potential for clinical translation. Pharmacol Ther. 2018;189:1‐21. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

