Non-Additive Effects of Binding Site Mutations in Calmodulin
- PMID: 31124357
- PMCID: PMC7909743
- DOI: 10.1021/acs.biochem.9b00096
Non-Additive Effects of Binding Site Mutations in Calmodulin
Abstract
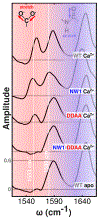
Despite decades of research on ion-sensing proteins, gaps persist in the understanding of ion binding affinity and selectivity even in well-studied proteins such as calmodulin. Site-directed mutagenesis is a powerful and popular tool for addressing outstanding questions about biological ion binding and is employed to selectively deactivate binding sites and insert chromophores at advantageous positions within ion binding structures. However, even apparently nonperturbative mutations can distort the binding dynamics they are employed to measure. We use Fourier transform infrared (FTIR) and ultrafast two-dimensional infrared (2D IR) spectroscopy of the carboxylate asymmetric stretching mode in calmodulin as a mutation- and label-independent probe of the conformational perturbations induced in calmodulin's binding sites by two classes of mutation, tryptophan insertion and carboxylate side-chain deletion, commonly used to study ion binding in proteins. Our results show that these mutations not only affect ion binding but also induce changes in calmodulin's conformational landscape along coordinates not probed by vibrational spectroscopy, remaining invisible without additional perturbation of binding site structure. Comparison of FTIR line shapes with 2D IR diagonal slices provides a clear example of how nonlinear spectroscopy produces well-resolved line shapes, refining otherwise featureless spectral envelopes into more informative vibrational spectra of proteins.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Forsen S, and Lindman B (1981) In Ion binding in biological systems as studied by NMR spectroscopy (Glick D, Ed.) Chapter 5, pp 289–486, John Wiley & Sons. - PubMed
-
- Roux B, Allen T, Berneche S, and Im W (2004) Theoretical and computational models of biological ion channels. Q. Rev. Biophys 37, 15–103. - PubMed
-
- Kilhoffer MC, Kubina M, Travers F, and Haiech J (1992) Use of engineered proteins with internal tryptophan reporter groups and perturbation techniques to probe the mechanism of ligand-protein interactions: investigation of the mechanism of calcium binding to calmodulin. Biochemistry 31, 8098–8106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

