Post-Ranibizumab injection endophthalmitis in aggressive posterior retinopathy of prematurity
- PMID: 31124535
- PMCID: PMC6552608
- DOI: 10.4103/ijo.IJO_884_17
Post-Ranibizumab injection endophthalmitis in aggressive posterior retinopathy of prematurity
Abstract
A preterm infant with zone 1 aggressive posterior retinopathy of prematurity developed infectious endophthalmitis after intravitreal injection of ranibizumab. Urgent empirical intravitreal therapy with vancomycin, ceftazidime, and dexamethasone along with intravenous therapy with amikacin and meropenem helped in early resolution. Vascularization/activity of disease subsided on follow-up, media cleared, and laser photocoagulation was completed. Later the disease reactivated, developed vitreous membranes and central retinal traction, for which 25-gauge lens-sparing vitrectomy was performed. Emergent treatment helped in salvaging the eye from both aggressive ROP disease and devastating endophthalmitis. Rationale approach to such a case is being discussed.
Keywords: Endophthalmitis; ranibizumab; retinopathy of prematurity; vascular endothelial growth factor.
Conflict of interest statement
None
Figures
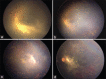
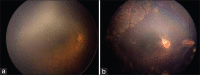
Comment in
-
Commentary: Antivascular endothelial growth factor and retinopathy of prematurity.Indian J Ophthalmol. 2019 Jun;67(6):969-970. doi: 10.4103/ijo.IJO_1220_17. Indian J Ophthalmol. 2019. PMID: 31124536 Free PMC article. No abstract available.
Similar articles
-
Characteristics of retinal vascularization in reactivated retinopathy of prematurity requiring treatment and clinical outcome after reinjection of ranibizumab.Sci Rep. 2024 Jul 8;14(1):15647. doi: 10.1038/s41598-024-66483-2. Sci Rep. 2024. PMID: 38977744 Free PMC article.
-
Retinopathy of Prematurity Reactivated 28 Months after Injection of Ranibizumab.Ophthalmol Retina. 2019 Oct;3(10):913-915. doi: 10.1016/j.oret.2019.06.017. Epub 2019 Jul 9. Ophthalmol Retina. 2019. PMID: 31474514 No abstract available.
-
[The clinical study on intravitreous injection of ranibizumab for aggressive posterior retinopathy of prematurity].Zhonghua Yan Ke Za Zhi. 2015 Nov;51(11):822-5. Zhonghua Yan Ke Za Zhi. 2015. PMID: 26850583 Chinese.
-
Antivascular endothelial growth factor in the treatment of retinopathy of prematurity.Curr Opin Ophthalmol. 2016 Sep;27(5):387-92. doi: 10.1097/ICU.0000000000000286. Curr Opin Ophthalmol. 2016. PMID: 27206263 Review.
-
Anti-Vascular Endothelial Growth Factor Therapy for Primary Treatment of Type 1 Retinopathy of Prematurity: A Report by the American Academy of Ophthalmology.Ophthalmology. 2017 May;124(5):619-633. doi: 10.1016/j.ophtha.2016.12.025. Epub 2017 Mar 22. Ophthalmology. 2017. PMID: 28341474 Review.
Cited by
-
Aggressive posterior retinopathy of prematurity: a review on current understanding.Eye (Lond). 2021 Apr;35(4):1140-1158. doi: 10.1038/s41433-021-01392-6. Epub 2021 Jan 29. Eye (Lond). 2021. PMID: 33514899 Free PMC article. Review.
-
The UK practice of Anti-VEGF therapy for treatment of retinopathy of prematurity.Eye (Lond). 2021 Sep;35(9):2451-2453. doi: 10.1038/s41433-021-01543-9. Epub 2021 Apr 20. Eye (Lond). 2021. PMID: 33879853 Free PMC article. No abstract available.
-
Commentary: Antivascular endothelial growth factor and retinopathy of prematurity.Indian J Ophthalmol. 2019 Jun;67(6):969-970. doi: 10.4103/ijo.IJO_1220_17. Indian J Ophthalmol. 2019. PMID: 31124536 Free PMC article. No abstract available.
-
Endophthalmitis in Retinopathy of Prematurity after Intravitreal Aflibercept Injection.Case Rep Ophthalmol Med. 2020 Dec 21;2020:8861435. doi: 10.1155/2020/8861435. eCollection 2020. Case Rep Ophthalmol Med. 2020. PMID: 33425413 Free PMC article.
-
Bedside bilateral sequential intravitreal anti-VEGF injections for retinopathy of prematurity.Indian J Ophthalmol. 2025 Jan 1;73(Suppl 1):S112-S118. doi: 10.4103/IJO.IJO_558_24. Epub 2024 Sep 10. Indian J Ophthalmol. 2025. PMID: 39257079 Free PMC article.
References
-
- McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: Causative organisms and possible prevention strategies. Retina. 2011;31:654–61. - PubMed
-
- Basu S, Kumar A, Kapoor K, Bagri NK, Chandra A. Neonatal endogenous endophthalmitis: A report of six cases. Pediatrics. 2013;131:e1292–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

