Phase 1 study of selinexor plus carfilzomib and dexamethasone for the treatment of relapsed/refractory multiple myeloma
- PMID: 31124580
- PMCID: PMC6772147
- DOI: 10.1111/bjh.15969
Phase 1 study of selinexor plus carfilzomib and dexamethasone for the treatment of relapsed/refractory multiple myeloma
Abstract
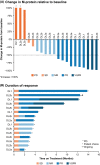
Selinexor, an oral Selective Inhibitor of Nuclear Export, targets Exportin 1 (XPO1, also termed CRM1). Non-clinical studies support combining selinexor with proteasome inhibitors (PIs) and corticosteroids to overcome resistance in relapsed/refractory multiple myeloma (RRMM). We conducted a phase I dose-escalation trial of twice-weekly selinexor in combination with carfilzomib and dexamethasone (SKd) to determine maximum tolerated dose in patients with RRMM (N = 21), with an expansion cohort to assess activity in carfilzomib-refractory disease and identify a recommended phase II dose (RP2D). During dose escalation, there was one dose-limiting toxicity (cardiac failure). The RP2D of twice-weekly SKd was selinexor 60 mg, carfilzomib 20/27 mg/m2 and dexamethasone 20 mg. The most common grade 3/4 treatment-emergent adverse events included thrombocytopenia (71%), anaemia (33%), lymphopenia (33%), neutropenia (33%) and infections (24%). Rates of ≥minimal response, ≥partial response and very good partial response were 71%, 48% and 14%, respectively; similar response outcomes were observed for dual-class refractory (PI and immunomodulatory drug)/quad-exposed (carfilzomib, bortezomib, lenalidomide and pomalidomide) patients (n = 17), and patients refractory to carfilzomib in last line of therapy (n = 13). Median progression-free survival was 3·7 months, and overall survival was 22·4 months in the overall population. SKd was tolerable and re-established disease control in RRMM patients, including carfilzomib-refractory patients. Registered at ClinicalTrials.gov (NCT02199665).
Keywords: carfilzomib; dexamethasone; relapsed/refractory multiple myeloma; selinexor.
© 2019 British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
A.J.J. has received research funding from Bristol‐Myers Squibb, Amgen and Karyopharm and has received personal fees (advisory board, consultancy, speaking and honoraria) from Bristol‐Myers Squibb, Celgene, Millennium, Novartis, Amgen, SkylineDx, Karyopharm and Sanofi‐Aventis. C.A.R. received research funding from Novartis, Millennium, GlaxoSmithKline and Bristol‐Meyers Squibb; received honoraria, travel accommodations and expenses from Bristol‐Myers Squibb and Celgene. C.E.C. received travel, accommodations and expenses from Amgen. A.C. holds a consultancy/advisory role for Amgen, Array Biopharma, Bristol‐Myers Squibb, Celgene, Janssen Oncology, Millennium and Novartis; received research funding from Acetylon Pharmaceuticals (Inst), Array BioPharma, Biotest (Inst), Bristol‐Myers Squibb (Inst), Celgene, Janssen, Millennium, Novartis, Onyx and Pharmacyclics LLC, an AbbVie Company; and received travel accommodations and expenses from Amgen, Bristol‐Myers Squibb, Celgene, Janssen Oncology, Novartis and Takeda. J.M. received research funding from AbbVie, Celgene and Sanofi. A.M. and L.A.S. are employed with and have ownership interests in TG Therapeutics. T.M.Z. is employed with AbbVie. J.A.Z. holds a consultancy/advisory role for Amgen, Array BioPharma, Bristol‐Myers Squibb, Celgene, Janssen, Prothena, Seattle Genetics and Takeda; and received research funding for Celgene (Inst). The remaining authors declare no competing financial interests.
Figures


References
-
- Bahlis, N.J. , Sutherland, H. , White, D. , Sebag, M. , Lentzsch, S. , Kotb, R. , Venner, C.P. , Gasparetto, C. , Del Col, A. , Neri, P. , Reece, D. , Kauffman, M. , Shacham, S. , Unger, T.J. , Jeha, J. , Saint‐Martin, J.R. , Shah, J. & Chen, C. (2018) Selinexor plus low‐dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood, 132, 2546–2554. - PMC - PubMed
-
- Berenson, J.R. , Hilger, J.D. , Yellin, O. , Dichmann, R. , Patel‐Donnelly, D. , Boccia, R.V. , Bessudo, A. , Stampleman, L. , Gravenor, D. , Eshaghian, S. , Nassir, Y. , Swift, R.A. & Vescio, R.A. (2014) Replacement of bortezomib with carfilzomib for multiple myeloma patients progressing from bortezomib combination therapy. Leukemia, 28, 1529–1536. - PubMed
-
- Berenson, J.R. , Cartmell, A. , Bessudo, A. , Lyons, R.M. , Harb, W. , Tzachanis, D. , Agajanian, R. , Boccia, R. , Coleman, M. , Moss, R.A. , Rifkin, R.M. , Patel, P. , Dixon, S. , Ou, Y. , Anderl, J. , Aggarwal, S. & Berdeja, J.G. (2016) CHAMPION‐1: a phase 1/2 study of once‐weekly carfilzomib and dexamethasone for relapsed or refractory multiple myeloma. Blood, 127, 3360–3368. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

