A ribosome assembly stress response regulates transcription to maintain proteome homeostasis
- PMID: 31124783
- PMCID: PMC6579557
- DOI: 10.7554/eLife.45002
A ribosome assembly stress response regulates transcription to maintain proteome homeostasis
Abstract
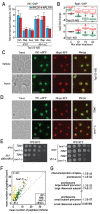
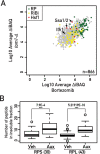
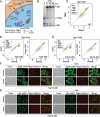
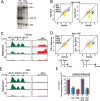
Ribosome biogenesis is a complex and energy-demanding process requiring tight coordination of ribosomal RNA (rRNA) and ribosomal protein (RP) production. Given the extremely high level of RP synthesis in rapidly growing cells, alteration of any step in the ribosome assembly process may impact growth by leading to proteotoxic stress. Although the transcription factor Hsf1 has emerged as a central regulator of proteostasis, how its activity is coordinated with ribosome biogenesis is unknown. Here, we show that arrest of ribosome biogenesis in the budding yeast Saccharomyces cerevisiae triggers rapid activation of a highly specific stress pathway that coordinately upregulates Hsf1 target genes and downregulates RP genes. Activation of Hsf1 target genes requires neo-synthesis of RPs, which accumulate in an insoluble fraction and presumably titrate a negative regulator of Hsf1, the Hsp70 chaperone. RP aggregation is also coincident with that of the RP gene activator Ifh1, a transcription factor that is rapidly released from RP gene promoters. Our data support a model in which the levels of newly synthetized RPs, imported into the nucleus but not yet assembled into ribosomes, work to continuously balance Hsf1 and Ifh1 activity, thus guarding against proteotoxic stress during ribosome assembly.
Keywords: Ifh1; S. cerevisiae; cell biology; chromosomes; gene expression; heat shock factor 1; protein aggregation; ribosomal protein; ribosome biogenesis; topoisomerase.
© 2019, Albert et al.
Conflict of interest statement
BA, IK, AH, CD, MR, XZ, OG, MK, DS No competing interests declared
Figures








References
-
- Brandman O, Stewart-Ornstein J, Wong D, Larson A, Williams CC, Li GW, Zhou S, King D, Shen PS, Weibezahn J, Dunn JG, Rouskin S, Inada T, Frost A, Weissman JS. A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell. 2012;151:1042–1054. doi: 10.1016/j.cell.2012.10.044. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

