Efficacy of 12-weeks velpatasvir plus sofosbuvir-based regimen in HCV-naive subjects with mild fibrosis: a meta-analysis
- PMID: 31124995
- PMCID: PMC6776220
- DOI: 10.23750/abm.v90i2.8374
Efficacy of 12-weeks velpatasvir plus sofosbuvir-based regimen in HCV-naive subjects with mild fibrosis: a meta-analysis
Abstract
Background and aims: In literature systematic data on treatment with the fixed-dose combination of sofosbuvir and velpatasvir for 12 weeks in anti-HCV/HCV RNA positive subjects with mild fibrosis and naïve to previous Interferon free regimen are scanty. A meta-analysis has been performed to evaluate the efficacy of velpatasvir plus sofosbuvir combination in these patients.
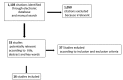
Methods: All randomized or non-randomized studies, investigating the sustained virological response rate to sofosbuvir plus velpatasvir without ribavirin for 12 weeks in subjects naïve to previous DAA therapy and with fibrosis F0-F2 or F0-F3, were included in the meta-analysis.
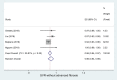
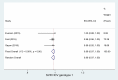
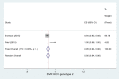
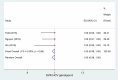
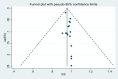
Results: A total of 16 studies enrolling 4,907 subjects met the inclusion criteria and were included in this meta-analysis. The prevalence of SVR by sofosbuvir and velpatasvir was 98% (95% CI 96-99%) in the 4,907 subjects without cirrhosis. The prevalence of SVR was similar considering the 9 clinical studies and the 7 real-world studies (98%, CI 95%: 96-99% and 98%; CI 95%: 96-99%, respectively). Considering the 4 studies enrolling 1,371 subjects without advanced liver fibrosis the prevalence of SVR was also high [96% (95% CI: 94-98%)]. Data indicate a prevalence of SVR ranging to 95-100% according to the different HCV genotypes.
Conclusion: Sofosbuvir plus velpatasvir therapeutic regimen was highly effective in HCV patients without advanced liver disease naïve to previous DAA regimen independently the different HCV genotypes.
Conflict of interest statement
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
Figures


Similar articles
-
Efficacy of the Combination of Sofosbuvir, Velpatasvir, and the NS3/4A Protease Inhibitor GS-9857 in Treatment-Naïve or Previously Treated Patients With Hepatitis C Virus Genotype 1 or 3 Infections.Gastroenterology. 2016 Sep;151(3):448-456.e1. doi: 10.1053/j.gastro.2016.05.021. Epub 2016 May 27. Gastroenterology. 2016. PMID: 27240903 Clinical Trial.
-
Efficacy and safety of sofosbuvir-velpatasvir with or without ribavirin in HCV-infected Japanese patients with decompensated cirrhosis: an open-label phase 3 trial.J Gastroenterol. 2019 Jan;54(1):87-95. doi: 10.1007/s00535-018-1503-x. Epub 2018 Sep 10. J Gastroenterol. 2019. PMID: 30203225 Free PMC article. Clinical Trial.
-
Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial.Lancet Gastroenterol Hepatol. 2016 Oct;1(2):122-132. doi: 10.1016/S2468-1253(16)30009-7. Epub 2016 Aug 3. Lancet Gastroenterol Hepatol. 2016. PMID: 28404069 Clinical Trial.
-
Sofosbuvir/Velpatasvir: The First Pangenotypic Direct-Acting Antiviral Combination for Hepatitis C.Ann Pharmacother. 2017 Jan;51(1):44-53. doi: 10.1177/1060028016668897. Epub 2016 Oct 1. Ann Pharmacother. 2017. PMID: 27609942 Review.
-
Sofosbuvir-velpatasvir: A single-tablet treatment for hepatitis C infection of all genotypes.Am J Health Syst Pharm. 2017 Jul 15;74(14):1045-1052. doi: 10.2146/ajhp60632. Am J Health Syst Pharm. 2017. PMID: 28687550 Review.
Cited by
-
Real-world treatment outcome of direct-acting antivirals and patient survival rates in chronic hepatitis C virus infection in Eritrea.Sci Rep. 2023 Nov 27;13(1):20792. doi: 10.1038/s41598-023-47258-7. Sci Rep. 2023. PMID: 38012181 Free PMC article.
-
Efficacy and Safety of Generic Sofosbuvir Plus Daclatasvir and Sofosbuvir/Velpatasvir in HCV Genotype 3-Infected Patients: Real-World Outcomes From Pakistan.Front Pharmacol. 2020 Sep 2;11:550205. doi: 10.3389/fphar.2020.550205. eCollection 2020. Front Pharmacol. 2020. PMID: 32982753 Free PMC article.
-
High Rate of Hepatitis C Virus Reinfection Among Recently Injecting Drug Users: Results From the TraP Hep C Program-A Prospective Nationwide, Population-Based Study.Clin Infect Dis. 2022 Nov 14;75(10):1732-1739. doi: 10.1093/cid/ciac272. Clin Infect Dis. 2022. PMID: 35438144 Free PMC article.
-
Sofosbuvir/Velpatasvir - A Promising Treatment for Chronic Hepatitis C Virus Infection.Cureus. 2021 Aug 16;13(8):e17237. doi: 10.7759/cureus.17237. eCollection 2021 Aug. Cureus. 2021. PMID: 34540464 Free PMC article. Review.
-
Efficacy and safety of direct-acting antiviral regimen for patients with hepatitis C virus genotype 2: a systematic review and meta-analysis.BMC Gastroenterol. 2024 Sep 30;24(1):331. doi: 10.1186/s12876-024-03414-5. BMC Gastroenterol. 2024. PMID: 39350091 Free PMC article.
References
-
- Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013 Apr;57:1333–42. - PubMed
-
- EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461–511. - PubMed
-
- AASLD-IDSA HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. https://www.hcvguidelines.org .
-
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. - PubMed
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

