Evaluation of Intussusception After Oral Monovalent Rotavirus Vaccination in South Africa
- PMID: 31125061
- PMCID: PMC7146001
- DOI: 10.1093/cid/ciz431
Evaluation of Intussusception After Oral Monovalent Rotavirus Vaccination in South Africa
Abstract
Background: Postlicensure studies have shown an association between rotavirus vaccination and intussusception. We assessed the risk of intussusception associated with Rotarix (RV1) administration, at 6 and 14 weeks of age, in an upper-middle-income country, South Africa.
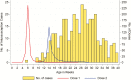
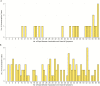
Methods: Active prospective surveillance for intussusception was conducted in 8 hospitals from September 2013 through December 2017. Retrospective case enrollment was done at 1 hospital from July 2012 through August 2013. Demographic characteristics, symptom onset, and rotavirus vaccine status were ascertained. Using the self-controlled case-series method, we estimated age-adjusted incidence rate ratios within 1-7, 8-21, and 1-21 days of rotavirus vaccination in children aged 28-275 days at onset of symptoms. In addition, age-matched controls were enrolled for a subset of cases (n = 169), and a secondary analysis was performed.
Results: Three hundred forty-six cases were included in the case-series analysis. Post-dose 1, there were zero intussusception cases within 1-7 days, and 5 cases within 8-21 days of vaccination. Post-dose 2, 15 cases occurred within 1-7 days, and 18 cases within 8-21 days of vaccination. There was no increased risk of intussusception 1-7 days after dose 1 (no cases observed) or dose 2 (relative incidence [RI], 1.71 [95% confidence interval {CI} .83-3.01]). Similarly, there was no increased risk 8-21 days after the first (RI, 4.01 [95% CI, .87-10.56]) or second dose (RI, .96 [95% CI, .52-1.60]). Results were similar for the case-control analysis.
Conclusions: The risk of intussusception in the 21 days after the first or second dose of RV1 was not higher than the background risk among South Africa infants.
Clinical trials registration: South African National Clinical Trial Register (DOH-27-0913-4183).
Keywords: infant; intussusception; rotavirus vaccine; safety.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- Mpabalwani EM, Mwenda JM, Tate JE, Parashar UD. Review of naturally occurring intussusception in young children in the WHO African region prior to the era of rotavirus vaccine utilization in the expanded programme of immunization. J Trop Pediatr 2017; 63:221–8. - PubMed
-
- Murphy TV, Gargiullo PM, Massoudi MS, et al. . Rotavirus Intussusception Investigation Team Intussusception among infants given an oral rotavirus vaccine. N Engl J Med 2001; 344:564–72. - PubMed
-
- Vesikari T, Matson DO, Dennehy P, et al. . Rotavirus Efficacy and Safety Trial (REST) Study Team Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23–33. - PubMed
-
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. . Human Rotavirus Vaccine Study Group Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

