Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry
- PMID: 31125062
- PMCID: PMC7389252
- DOI: 10.1093/annonc/mdz167
Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry
Abstract
Background: Anti-PD1/PD-L1 directed immune checkpoint inhibitors (ICI) are widely used to treat patients with advanced non-small-cell lung cancer (NSCLC). The activity of ICI across NSCLC harboring oncogenic alterations is poorly characterized. The aim of our study was to address the efficacy of ICI in the context of oncogenic addiction.
Patients and methods: We conducted a retrospective study for patients receiving ICI monotherapy for advanced NSCLC with at least one oncogenic driver alteration. Anonymized data were evaluated for clinicopathologic characteristics and outcomes for ICI therapy: best response (RECIST 1.1), progression-free survival (PFS), and overall survival (OS) from ICI initiation. The primary end point was PFS under ICI. Secondary end points were best response (RECIST 1.1) and OS from ICI initiation.
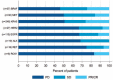
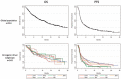
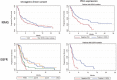
Results: We studied 551 patients treated in 24 centers from 10 countries. The molecular alterations involved KRAS (n = 271), EGFR (n = 125), BRAF (n = 43), MET (n = 36), HER2 (n = 29), ALK (n = 23), RET (n = 16), ROS1 (n = 7), and multiple drivers (n = 1). Median age was 60 years, gender ratio was 1 : 1, never/former/current smokers were 28%/51%/21%, respectively, and the majority of tumors were adenocarcinoma. The objective response rate by driver alteration was: KRAS = 26%, BRAF = 24%, ROS1 = 17%, MET = 16%, EGFR = 12%, HER2 = 7%, RET = 6%, and ALK = 0%. In the entire cohort, median PFS was 2.8 months, OS 13.3 months, and the best response rate 19%. In a subgroup analysis, median PFS (in months) was 2.1 for EGFR, 3.2 for KRAS, 2.5 for ALK, 3.1 for BRAF, 2.5 for HER2, 2.1 for RET, and 3.4 for MET. In certain subgroups, PFS was positively associated with PD-L1 expression (KRAS, EGFR) and with smoking status (BRAF, HER2).
Conclusions: : ICI induced regression in some tumors with actionable driver alterations, but clinical activity was lower compared with the KRAS group and the lack of response in the ALK group was notable. Patients with actionable tumor alterations should receive targeted therapies and chemotherapy before considering immunotherapy as a single agent.
Keywords: immunotherapy; lung cancer; oncogenic addiction.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Immune checkpoint inhibitors and driver oncogenes in non-small cell lung cancer.Transl Cancer Res. 2019 Dec;8(Suppl 6):S628-S632. doi: 10.21037/tcr.2019.10.08. Transl Cancer Res. 2019. PMID: 35117146 Free PMC article. No abstract available.
References
-
- Doroshow D.B., Herbst R.S. Treatment of advanced non-small cell lung cancer in 2018. JAMA Oncol. 2018;4(4):569–570. - PubMed
-
- Resources N. NCCN Framework for Resource Stratification of NCCN Guidelines (NCCN Framework™) 2019 https://www.nccn.org/framework/
-
- Reck M., Rodriguez-Abreu D., Robinson A.G. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. - PubMed
-
- Socinski M.A., Jotte R.M., Cappuzzo F. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

