Deoxynucleoside Therapy for Thymidine Kinase 2-Deficient Myopathy
- PMID: 31125140
- PMCID: PMC7586249
- DOI: 10.1002/ana.25506
Deoxynucleoside Therapy for Thymidine Kinase 2-Deficient Myopathy
Abstract
Objective: Thymidine kinase 2, encoded by the nuclear gene TK2, is required for mitochondrial DNA maintenance. Autosomal recessive TK2 mutations cause depletion and multiple deletions of mtDNA that manifest predominantly as a myopathy usually beginning in childhood and progressing relentlessly. We investigated the safety and efficacy of deoxynucleoside monophosphate and deoxynucleoside therapies.
Methods: We administered deoxynucleoside monophosphates and deoxynucleoside to 16 TK2-deficient patients under a compassionate use program.
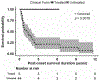
Results: In 5 patients with early onset and severe disease, survival and motor functions were better than historically untreated patients. In 11 childhood and adult onset patients, clinical measures stabilized or improved. Three of 8 patients who were nonambulatory at baseline gained the ability to walk on therapy; 4 of 5 patients who required enteric nutrition were able to discontinue feeding tube use; and 1 of 9 patients who required mechanical ventilation became able to breathe independently. In motor functional scales, improvements were observed in the 6-minute walk test performance in 7 of 8 subjects, Egen Klassifikation in 2 of 3, and North Star Ambulatory Assessment in all 5 tested. Baseline elevated serum growth differentiation factor 15 levels decreased with treatment in all 7 patients tested. A side effect observed in 8 of the 16 patients was dose-dependent diarrhea, which did not require withdrawal of treatment. Among 12 other TK2 patients treated with deoxynucleoside, 2 adults developed elevated liver enzymes that normalized following discontinuation of therapy.
Interpretation: This open-label study indicates favorable side effect profiles and clinical efficacy of deoxynucleoside monophosphate and deoxynucleoside therapies for TK2 deficiency. ANN NEUROL 2019;86:293-303.
© 2019 American Neurological Association.
Conflict of interest statement
Potential Conflicts of Interest
C.G., R.M., and M.H. are paid consultants to Modis Therapeutics. R.M. has equity in Modis Therapeutics. These relationships are de minimus for the United Kingdom Medical Research Council (C.G.), Vall d’Hebron Research Institute (R.M.), and Columbia University Medical Center (M.H.). Columbia University has submitted a patent, which has been licensed to Modis Therapeutics; this relationship is monitored by an unconflicted external academic researcher. The other authors declare no conflicts of interest.
Figures

References
-
- Berk AJ, Clayton DA. A genetically distinct thymidine kinase in mammalian mitochondria. Exclusive labeling of mitochondrial deoxyribonucleic acid. J Biol Chem 1973;248:2722–2729. - PubMed
-
- Wang L, Munch-Petersen B, Herrstrom Sjoberg A, et al. Human thymidine kinase 2: molecular cloning and characterisation of the enzyme activity with antiviral and cytostatic nucleoside substrates. FEBS Lett 1999;443:170–174. - PubMed
-
- Behin A, Jardel C, Claeys KG, et al. Adult cases of mitochondrial DNA depletion due to TK2 defect: an expanding spectrum. Neurology 2012;78:644–648. - PubMed
-
- Saada A, Shaag A, Mandel H, et al. Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nat Genet 2001; 29:342–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Arturo Estopinan TK2 Research Fund/International
- CP09/00011/Spanish Carlos III Health Institute/International
- Carlos III Health Institute/International
- U54 NS078059/NS/NINDS NIH HHS/United States
- PI16/00579/Spanish Carlos III Health Institute/International
- P01 HD080642/HD/NICHD NIH HHS/United States
- SLT002/16/00370/Generalitat de Catalunya PERIS program/International
- European Regional Development Fund/International
- 577391/Muscular Dystrophy Association/International
- PMP15/00025/Spanish Carlos III Health Institute/International
- Instituto de Salud Carlos III/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

